In this issue of Blood, Ge et al demonstrate a novel link between coagulation and inflammation by showing that factor XIa (FXIa) can generate the active chemoattractant and adipokine chemerin from its inactive precursor prochemerin.1
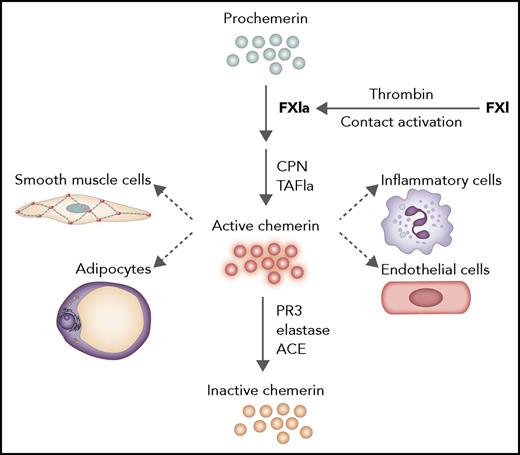
Processing of prochemerin by FXIa. FXI can be activated by contact activation or thrombin. FXIa can then process prochemerin to a form that can be modified by carboxypeptidase N (CPN) or activated thrombin-activatable fibrinolysis inhibitor to active chemerin. Chemerin is proinflammatory and acts as classical adipokine and chemokine on multiple cell types. Inactivation of chemerin occurs via proteolytic cleavage by proteinase 3, elastase, and angiotensin-converting enzyme. ACE, angiotensin-converting enzyme; PR3, proteinase 3; TAFIa, activated thrombin-activatable fibrinolysis inhibitor. Professional illustration by Somersault18:24.
Processing of prochemerin by FXIa. FXI can be activated by contact activation or thrombin. FXIa can then process prochemerin to a form that can be modified by carboxypeptidase N (CPN) or activated thrombin-activatable fibrinolysis inhibitor to active chemerin. Chemerin is proinflammatory and acts as classical adipokine and chemokine on multiple cell types. Inactivation of chemerin occurs via proteolytic cleavage by proteinase 3, elastase, and angiotensin-converting enzyme. ACE, angiotensin-converting enzyme; PR3, proteinase 3; TAFIa, activated thrombin-activatable fibrinolysis inhibitor. Professional illustration by Somersault18:24.
The processes of innate immunity and hemostasis are intimately intertwined.2 From an evolutionary perspective, and still seen in the horseshoe crab, both mechanisms originate from a single method to restrict and fight infections after injury to the organism. By capturing the pathogen in a clot, the intruder will be contained, and bleeding will be minimal. There is a lot of cross-talk between inflammation and hemostasis; coagulation markedly affects the inflammatory response, and proinflammatory cytokines and chemokines initiate and propagate coagulation activity.
Ge et al focus on the chemoattractant and adipokine chemerin (retinoic acid receptor responder gene 2). Chemerin is a proinflammatory molecule mainly expressed by white adipose tissue and the liver. Chemerin acts on a variety of cells (see figure) and is implicated in the inflammatory response, for instance, in psoriasis, metabolic syndrome, and cardiovascular disease.3 Chemerin circulates as an inactive precursor prochemerin in plasma and needs to be converted by proteolysis into one of several active species. Biological activities of these species are regulated by further proteolytic cleavages to inactive chemerin forms. First, by screening a panel of enzymes from the coagulation, fibrinolytic, and inflammation cascades, Ge et al establish that FXIa specifically generates a chemerin form that can be further processed into active chemerin by carboxypeptidases in the blood (CPN and activated thrombin-activatable fibrinolysis inhibitor). Strikingly, plasma kallikrein, which is highly homologous to FXIa, was very inefficient in processing prochemerin. The authors used a unique set of tools that specifically detected the different forms of chemerin, and they established that during activation of coagulation, prochemerin was processed, which coincided with the presence of FXIa. Processing of prochemerin in plasma was completely dependent on factor XI (FXI), because it did not occur in FXI–depleted plasma. The importance of FXI for prochemerin processing was confirmed in FXI–deficient patients, in whom the level of (intact) prochemerin was inversely associated with FXI levels in plasma. The action of FXIa was independent from its mode of activation, either by factor XIIa during contact activation or via feedback activation by thrombin. Taking all the data together, Ge et al’s article demonstrates the importance of prochemerin processing by FXIa in plasma and provides a novel link between coagulation and inflammation.
FXI has been implicated before in inflammation. Recently, it was demonstrated that thrombin-mediated FXI activation on platelets amplified thrombin generation that promoted vascular inflammation, resulting in arterial hypertension.4 In addition, both beneficial and detrimental effects of FXI were observed in infectious sepsis models in animals.5,6 In the models that were used, sepsis originated from different initial infection sites with different pathogens, suggesting that the local interaction between host and microbe has a decisive role in the outcome. What these studies had in common was that FXI was thought to influence inflammation by its role in the coagulation cascade or by direct actions on neutrophils.5,7 Ge et al now provide for the first time a different mechanism, namely, the processing of a chemokine that affects inflammation.
It is striking that the processing of prochemerin requires FXIa and a circulating carboxypeptidase. Both CPN and activated thrombin-activatable fibrinolysis inhibitor can act on chemerin. CPN is a constitutively active enzyme, whereas activated thrombin-activatable fibrinolysis inhibitor (also known as plasma carboxypeptidase B2) needs to be formed from its precursor by thrombin. This latter process is very much dependent on the feedback activation of FXI by thrombin,8 indicating a pivotal role of FXIa in regulation of chemerin bioactivity in plasma.
Targeting FXI has recently become a hot topic for the treatment of both venous and arterial diseases, because it reduces the thrombotic burden with minimal (or no) bleeding side effects. A reduction in thrombus formation already results in decreased inflammation, but it is attractive to postulate that in the case of FXI targeting, there is an additional beneficial effect of impaired chemerin-induced inflammatory responses. The clinical studies that are currently performed to establish efficacy and safety of FXI targeting may provide confirmation of this hypothesis.
Conflict-of-interest disclosure: Sanquin has received consultancy fees from Bayer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal