Studies investigating how micronutrients regulate erythropoiesis within the erythroblastic niche have primarily focused on iron homeostasis. In this issue of Blood, Liao et al highlight an additional regulator of the erythroblastic niche, selenium (Se).1 Se acts to promote stress erythroid cell expansion and differentiation as well as directly acting on monocytes, inducing their differentiation to erythroblastic island macrophages.
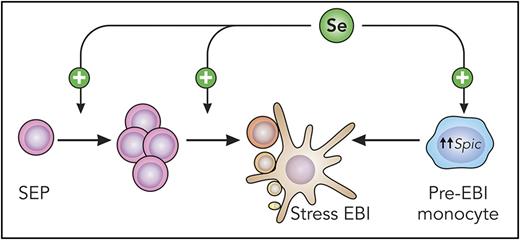
Schematic representation of Se function during stress erythropoiesis. Mouse stress erythroid progenitors (SEP) migrate from the bone marrow to the spleen, where Se acts to increase SEP numbers and differentiation to erythroid precursors. Optimal precursor maturation relies on the formation of functional EBIs. Se promotes the differentiation of island macrophages by upregulating Spic expression in monocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
Schematic representation of Se function during stress erythropoiesis. Mouse stress erythroid progenitors (SEP) migrate from the bone marrow to the spleen, where Se acts to increase SEP numbers and differentiation to erythroid precursors. Optimal precursor maturation relies on the formation of functional EBIs. Se promotes the differentiation of island macrophages by upregulating Spic expression in monocytes. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inadequate micronutrient intake causes anemia in ∼30% of the world population,2 and micronutrient deficiencies commonly associated with anemia include vitamin B12, folate, and iron. An overlooked cause of anemia is low levels of Se. How Se deficiency causes anemia in humans has eluded investigators because Se deficiency often occurs in the setting of multiple micronutrient abnormalities, such as patients with chronic inflammation/disease or patients on total parenteral nutrition.3,4 Therefore, Se studies have relied heavily on controlled experimental systems, such as mice and in vitro differentiation cultures.
Se engages in oxidation-reduction reactions through its incorporation into the selenocysteine-containing family of selenoproteins.5 Almost all selenoproteins possess antioxidant activities, which likely guided previous research to focus on the relationship between Se and oxidative stress during erythropoiesis. Indeed, genetic approaches and dietary deprivation of Se in mice were shown to lead to an increase in red cell reactive oxygen species and subsequent hemolysis, thereby confirming its antioxidant activity.6,7 Of note, Paulson and Prabhu made an observation that diverged from prevailing notions regarding selenoproteins. Se-deficient mice possessed elevated erythroid progenitors and lacked compensatory reticulocytosis, suggesting that Se exerts a broader effect, much earlier in erythroid differentiation than at the mature red blood cell stage, as it was previously hypothesized.
These initial findings serve as the basis for the work presented in this issue of Blood. Liao et al elucidate the mechanisms by which Se regulates stress erythropoiesis and also identifies an intriguing new function for Se in the formation of stress erythroblastic islands (EBI)1 (see figure). By inducing a loss of selenoproteins in vivo, the authors demonstrate an ineffective stress response as a result of reduced stress erythroid progenitor numbers and differentiation. Loss of selenoproteins also abrogates the differentiation of stress erythroid precursors at the proerythroblast (ProE) to basophilic erythroblast (BasoE) transition. Investigation of transcriptome-wide changes in ProE and BasoE identified selenoprotein W (SelenoW) as a novel regulator of stress erythropoiesis in mice and humans. The master erythroid transcription factor GATA1 drives SelenoW expression, and in addition to its antioxidant activity, SelenoW modulates cell signaling pathways necessary for stress erythropoiesis, suchas the Hippo pathway.8 Because SelenoW acts as a positive regulator of Hippo signaling at the level of the transcription factor Taz, SelenoW could represent a new therapeutic target for stimulating stress erythropoiesis.
An unexpected discovery from this work is that Se influences erythroid niche development in the spleen, where stress erythropoiesis predominantly occurs in the mouse. EBIs are characterized by terminally differentiating erythroid precursors invested by a single central macrophage.9 Selenoprotein deficiency affects macrophage differentiation from monocytes due to altered Spic expression and heme homeostasis, and the resultant decrease in red pulp macrophages correlates with quantitative and qualitative diminution of stress EBIs.
This exciting work answers long-standing mechanistic questions regarding the role of Se in stress erythropoiesis, which may carry clinical implications, particularly, for anemia of inflammation/chronic disease. In this context, where micronutrient handling by the body, including iron, is perturbed, Se supplementation could be considered. Nevertheless, before translating these findings more broadly to humans, many questions remain. For example, anatomic differences exist between murine and human stress erythropoiesis. In humans, stress responses mainly occur in the bone marrow, whereas stress erythropoiesis occurs in the spleen of mice. Do these anatomical differences reflect differences in the composition and function of stress EBIs? Adding to this complexity, the identity of the central macrophage during steady-state erythropoiesis remains unclear. As we witness a dramatic increase in the interest of EBIs in the field of erythropoiesis, we are certain that the differences between steady state and stress EBIs, highlighted by their differing reliance on Se, will ignite further exploration into understanding these niches.
Conflict-of-interest disclosure: The authors declare no competing financial interests.