Key Points
PK deficiency manifests a broad spectrum in anemia severity that moderately improves after splenectomy.
Close attention to monitoring for iron overload, gallstones, and other complications is recommended in all patients with PK deficiency.
Abstract
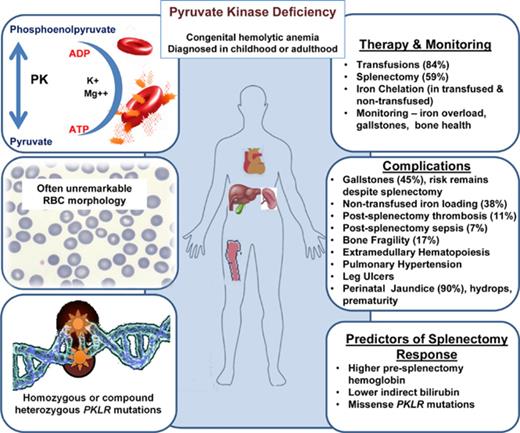
An international, multicenter registry was established to collect retrospective and prospective clinical data on patients with pyruvate kinase (PK) deficiency, the most common glycolytic defect causing congenital nonspherocytic hemolytic anemia. Medical history and laboratory and radiologic data were retrospectively collected at enrollment for 254 patients with molecularly confirmed PK deficiency. Perinatal complications were common, including anemia that required transfusions, hyperbilirubinemia, hydrops, and prematurity. Nearly all newborns were treated with phototherapy (93%), and many were treated with exchange transfusions (46%). Children age 5 years and younger were often transfused until splenectomy. Splenectomy (150 [59%] of 254 patients) was associated with a median increase in hemoglobin of 1.6 g/dL and a decreased transfusion burden in 90% of patients. Predictors of a response to splenectomy included higher presplenectomy hemoglobin (P = .007), lower indirect bilirubin (P = .005), and missense PKLR mutations (P = .0017). Postsplenectomy thrombosis was reported in 11% of patients. The most frequent complications included iron overload (48%) and gallstones (45%), but other complications such as aplastic crises, osteopenia/bone fragility, extramedullary hematopoiesis, postsplenectomy sepsis, pulmonary hypertension, and leg ulcers were not uncommon. Overall, 87 (34%) of 254 patients had both a splenectomy and cholecystectomy. In those who had a splenectomy without simultaneous cholecystectomy, 48% later required a cholecystectomy. Although the risk of complications increases with severity of anemia and a genotype-phenotype relationship was observed, complications were common in all patients with PK deficiency. Diagnostic testing for PK deficiency should be considered in patients with apparent congenital hemolytic anemia and close monitoring for iron overload, gallstones, and other complications is needed regardless of baseline hemoglobin. This trial was registered at www.clinicaltrials.gov as #NCT02053480.
Introduction
Red cell pyruvate kinase (PK) deficiency is a rare congenital, nonspherocytic hemolytic anemia caused by a glycolytic defect that is due to compound heterozygous or homozygous mutations in the PKLR gene on chromosome 1q21.1 PK catalyzes the conversion of phosphoenolpyruvate to pyruvate, creating red cell adenosine triphosphate. PK deficiency leads to a reduction in ATP and shortened reticulocyte and red cell lifespan because of an inability to maintain the red cell electrochemical gradient and membrane integrity as well as red cell damage and clearance in the spleen.2,3
The exact prevalence of PK deficiency is not known, although earlier reports have estimated that the prevalence of heterozygous carriers ranges from 1% to 3%, and the prevalence of the disease is 1 per 20 000 in the white population.4,5 There is a high frequency of PK deficiency among the Pennsylvania Amish community as a result of a founder effect.6 Because of the rarity of PK deficiency, its clinical features have been reported in previous case series mainly at single centers.7-10 Clinical features vary widely, ranging from a mildly symptomatic anemia to one that necessitates regular transfusions. The frequency of complications such as jaundice, gallstones, iron overload, severe anemia, thrombosis, and osteopenia is not well understood and has not been studied in a large population of patients with PK deficiency.11
Currently, there are no guidelines for the diagnosis, management, and monitoring of children and adults with PK deficiency. Best practice with regard to transfusion regimen and splenectomy is not known. Management strategies often emulate those of patients with hereditary spherocytosis or thalassemia intermedia, because it is not clear how patients with PK deficiency differ in terms of their risk of complications or clinical course.
The Pyruvate Kinase Deficiency Natural History Study was developed in 2013 with a retrospective and prospective longitudinal cohort to characterize the clinical manifestations, complications, and treatments of patients to support and guide the development of future treatments for PK deficiency.
Methods
Patients
Eligible patients had biochemically or molecularly diagnosed PK deficiency. If patients had prior genetic testing that confirmed 2 pathogenic PKLR mutations, the testing was not repeated. If prior genetic testing was not conducted or the results were not available, samples were sent to Ca’ Granda Ospedale Maggiore Policlinico Milan or Yale-New Haven Children’s Hospital for molecular analysis by Sanger sequencing. To evaluate genotype-phenotype associations, patients (n = 193) were grouped according to genotype (2 missense [M/M], 1 missense/1 nonmissense [M/NM], or 2 nonmissense mutations [NM/NM]); NM included nonsense, frameshift, inframe small indel, large deletions, and splicing mutations. Patients with 3 pathogenic variants (n = 2) or promoter mutations with unknown pathogenic effects (n = 4) were excluded from the genotype-phenotype analysis. Patients from the Amish community (homozygous for the splicing mutation R479H [n = 55]) were also excluded from the genotype-phenotype analysis.
The study protocol was approved by each site’s institutional review board and/or ethics committee, and all patients gave informed consent. Patients were able to participate from a distance by using signed medical releases or were primarily observed at a center approved to conduct the study. At the time of enrollment, patients’ medical records were reviewed retrospectively. Data collected included medical history, examination findings, and laboratory and radiologic studies. Medical history missing from the medical records was obtained by patient recall if known. All transfusion data were collected from medical records. At all sites, except in Lancaster, Pennsylvania, data were collected only if they were obtained for clinical purposes. In Lancaster, in which all enrolled patients were Amish, additional laboratory and radiologic data were collected under a site-specific institutional review board–approved protocol.
Statistical analysis
Patient demographics, transfusion status, comorbid diagnoses, and other disease characteristics were described with frequencies, proportions, medians, means, ranges, and interquartile ranges as appropriate for sample size. Tests of association were performed using Fisher’s exact test, the Wilcoxon rank sum test, or Kruskal-Wallis test. Univariate ordinal logistic regression modeling was performed to identify factors predictive of clinical severity classification. The normalized PK activity was calculated as [(PKobs − PKLL) × 100]/(PKUL − PKLL) where PKobs is the observed PK enzyme value, and PKLL and PKUL are the lower limit and upper limit of the reference range, respectively. Regular transfusions were defined as having ≥6 transfusions over a 12-month period. When actual dates were unknown, an approximate date was reported. The description of the transfusion schedules was stratified by age, in which patients younger than age 5 years at enrollment were considered separately from patients age 5 years and older at enrollment. Sample sizes are presented for those with known data available for each variable. P values were two-sided, and P values < .05 were considered statistically significant. For the tests in the genotype-phenotype analysis, adjustment for multiple comparisons was performed by applying a significance level of P < .0055 instead of .05.
Results
Demographics
This study enrolled 278 patients with PK deficiency from June 2014 through April 2017 at 31 centers in 5 countries. After exclusion of ineligible patients because 2 pathogenic PKLR mutations could not be confirmed, there were 254 eligible participants. Baseline demographic information is presented in Table 1. Patients from the Amish community (n = 55) composed 22% of the total population. Thirty-five percent of patients were related to other participants in the study.
Demographic features and common complications of enrolled patients
| Characteristics . | All (N = 254*) . | Amish (n = 55) . | Non-Amish (n = 199) . | |||
|---|---|---|---|---|---|---|
| n . | . | n . | . | n . | . | |
| Sex | ||||||
| Male | 124 | 49% | 14 | 25% | 110 | 55% |
| Female | 130 | 51% | 41 | 75% | 89 | 45% |
| Age at diagnosis, y (range) | 243† | 0.4 (0-60.3) | 52 | 0 (0-10) | 191 | 1.1 (0-60.3) |
| Age at enrollment, y (range) | ||||||
| Overall | 254 | 19.0 (0.1-69.9) | 55 | 23.4 (0.2-60) | 199 | 17.6 (0.1-69.9) |
| <18 | 123 | 6.4 (0.1-17.7) | 22 | 7.4 (0.2-16.4) | 101 | 6.1 (0.1-17.7) |
| ≥18 | 131 | 36.2 (18.0-69.9) | 33 | 39.8 (18-60.4) | 98 | 35.4 (18.2-69.9) |
| White race | 235 | 92.5% | 55 | 100% | 180 | 90% |
| Hispanic ethnicity | 18 | 7.1% | 0 | 0% | 18 | 9% |
| Median number of lifetime transfusions (range) | 191 | 18 (1-516) | 39 | 12 (1-153) | 152 | 25 (1-516) |
| Splenectomized | 150 | 59% | 51 | 93% | 99 | 50% |
| Gallstones | 112/248 | 45% | 24 | 10% | 88 | 35% |
| Extramedullary hematopoiesis | 23/254 | 9% | 15 | 27% | 8 | 4% |
| Hepatic | 14 | 11 | 3 | |||
| Splenic | 14 | 11 | 3 | |||
| Paraspinous | 8 | 7 | 1 | |||
| Mediastinal | 7 | 7 | 0 | |||
| Pulmonary hypertension | 8/237 | 3% | 1 | 2% | 7 | 4% |
| Leg ulcers | 4/240 | 2% | 0 | 0% | 4 | 2% |
| Aplastic crises | 34/245 | 14% | 6 | 11% | 28 | 15% |
| Liver cirrhosis | 8/240 | 3% | 2 | 4% | 6 | 3% |
| Bone fracture | 41/239 | 17% | 7 | 13% | 34 | 18% |
| Endocrine dysfunction | ||||||
| Thyroid disease | 11/241 | 5% | 3 | 6% | 8 | 4% |
| Growth hormone deficiency | 6/236 | 3% | 0 | 0% | 6 | 3% |
| Hypoparathyroidism | 3/232 | 1% | 1 | 2% | 2 | 3% |
| Diabetes | 2/250 | 1% | 0 | 0% | 2 | 1% |
| Hypogonadal hypogonadism | 0/233 | 0% | 0 | 0% | 0 | 0% |
| Post pubertal | 148/251 | 59% | 39 | 71% | 109 | 56% |
| Normal puberty | 128/138‡ | 94% | 35 | 97% | 94 | 92% |
| Hormones administered | 2/115‡ | 2% | 0 | 0% | 2 | 2% |
| Slowed/delayed puberty | 9/118‡ | 8% | 1 | 3% | 8 | 9% |
| Characteristics . | All (N = 254*) . | Amish (n = 55) . | Non-Amish (n = 199) . | |||
|---|---|---|---|---|---|---|
| n . | . | n . | . | n . | . | |
| Sex | ||||||
| Male | 124 | 49% | 14 | 25% | 110 | 55% |
| Female | 130 | 51% | 41 | 75% | 89 | 45% |
| Age at diagnosis, y (range) | 243† | 0.4 (0-60.3) | 52 | 0 (0-10) | 191 | 1.1 (0-60.3) |
| Age at enrollment, y (range) | ||||||
| Overall | 254 | 19.0 (0.1-69.9) | 55 | 23.4 (0.2-60) | 199 | 17.6 (0.1-69.9) |
| <18 | 123 | 6.4 (0.1-17.7) | 22 | 7.4 (0.2-16.4) | 101 | 6.1 (0.1-17.7) |
| ≥18 | 131 | 36.2 (18.0-69.9) | 33 | 39.8 (18-60.4) | 98 | 35.4 (18.2-69.9) |
| White race | 235 | 92.5% | 55 | 100% | 180 | 90% |
| Hispanic ethnicity | 18 | 7.1% | 0 | 0% | 18 | 9% |
| Median number of lifetime transfusions (range) | 191 | 18 (1-516) | 39 | 12 (1-153) | 152 | 25 (1-516) |
| Splenectomized | 150 | 59% | 51 | 93% | 99 | 50% |
| Gallstones | 112/248 | 45% | 24 | 10% | 88 | 35% |
| Extramedullary hematopoiesis | 23/254 | 9% | 15 | 27% | 8 | 4% |
| Hepatic | 14 | 11 | 3 | |||
| Splenic | 14 | 11 | 3 | |||
| Paraspinous | 8 | 7 | 1 | |||
| Mediastinal | 7 | 7 | 0 | |||
| Pulmonary hypertension | 8/237 | 3% | 1 | 2% | 7 | 4% |
| Leg ulcers | 4/240 | 2% | 0 | 0% | 4 | 2% |
| Aplastic crises | 34/245 | 14% | 6 | 11% | 28 | 15% |
| Liver cirrhosis | 8/240 | 3% | 2 | 4% | 6 | 3% |
| Bone fracture | 41/239 | 17% | 7 | 13% | 34 | 18% |
| Endocrine dysfunction | ||||||
| Thyroid disease | 11/241 | 5% | 3 | 6% | 8 | 4% |
| Growth hormone deficiency | 6/236 | 3% | 0 | 0% | 6 | 3% |
| Hypoparathyroidism | 3/232 | 1% | 1 | 2% | 2 | 3% |
| Diabetes | 2/250 | 1% | 0 | 0% | 2 | 1% |
| Hypogonadal hypogonadism | 0/233 | 0% | 0 | 0% | 0 | 0% |
| Post pubertal | 148/251 | 59% | 39 | 71% | 109 | 56% |
| Normal puberty | 128/138‡ | 94% | 35 | 97% | 94 | 92% |
| Hormones administered | 2/115‡ | 2% | 0 | 0% | 2 | 2% |
| Slowed/delayed puberty | 9/118‡ | 8% | 1 | 3% | 8 | 9% |
Countries (No. of patients enrolled) were United States (154), Germany (31), Italy (25), The Netherlands (23), Canada (14), and Czech Republic (7).
N, total number of patients with data available for the characteristic; n, number of patients per demographic characteristic.
Some denominators are less than 254 because some patients had unknown data.
Eleven patients had unknown age at diagnosis.
One hundred forty-eight patients were post pubertal; however, some patients had unknown data.
The median normalized PK enzyme activity level was −69% (range, −486% to 118% [n = 107]), with 92 patients (86%) categorized as low, 14 (13%) as normal, and 1 (1%) as having high PK enzyme activity.
Perinatal complications
Before birth, when patients with PK deficiency were in utero, perinatal complications were reported in 28% (65 of 233; supplemental Table 1, available on the Blood Web site). For these 65 patients, preterm birth (56%), prenatal anemia requiring in utero transfusions (47%), and preterm labor (42%) were reported most commonly. Intrauterine growth retardation and/or fetal distress was reported in 24% of pregnancies, and 16% were complicated by hydrops. One newborn developed severe hepatic disease with evolution to liver failure. The majority (207 [90%] of 230) of newborns with PK deficiency developed an indirect hyperbilirubinemia. Jaundice was treated with phototherapy (93%) and/or exchange transfusion (46%).
Clinical complications
Comorbid diagnoses and complications are listed in Table 1. A history of aplastic crisis was reported in a small subset of patients (34 [14%] of 245). Extramedullary hematopoiesis was reported in 23 patients, with a significantly higher rate in Amish compared with non-Amish patients (P < .0001). There was no report of myelodysplastic syndrome. Leg ulcers were reported in 4 patients age 27 to 42 years at enrollment. Pulmonary hypertension occurred in 8 patients at a median age of 39.2 years (range, 0-42.6 years). The majority of these patients (7 of 8) had been splenectomized, and 1 was receiving regular transfusions at the time of enrollment. At enrollment, 1% of patients had a previous hepatitis B infection, and 3% had a previous hepatitis C infection.
Bone fractures were reported in 17% of patients. The median 25-hydroxyvitamin D level was 22 ng/mL (range, 9-145 ng/mL). A small number of patients had endocrine dysfunction; however, hormone levels were not uniformly monitored in this cohort.
On physical examination, facial jaundice (32%), scleral icterus (58%), bone deformities (9%), and hyperpigmentation (6%) were noted. Splenomegaly was reported in 35% of nonsplenectomized patients.
Surgical treatments
Splenectomy.
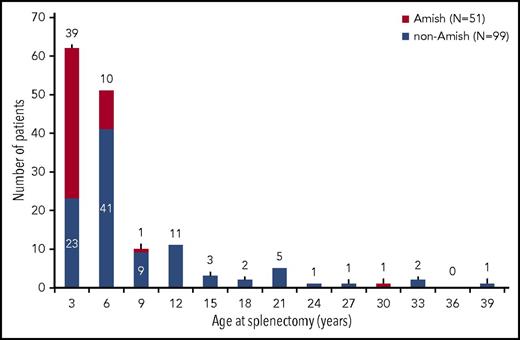
The majority of enrolled patients had a prior splenectomy (150 [59%] of 254), with a higher rate in Amish compared with non-Amish patients (P < .0001). Characteristics of the patients by splenectomy status and age distribution at the time of splenectomy are provided in Table 2 and Figure 1. The most common reasons for splenectomy (according to medical record review) were to improve baseline anemia (88%), decrease transfusion burden (85%), improve patient quality of life (83%), and reduce jaundice (57%). In 90% of patients, the transfusion burden was reduced after splenectomy. The reported median change in hemoglobin from pre- to postsplenectomy was 1.6 g/dL (range, −5.5 to 4.9 g/dL [n = 72]), with a reduction in hemoglobin noted in some patients when transfusions were discontinued.
Response to splenectomy
| . | Response to splenectomy . | P* . | ||
|---|---|---|---|---|
| No response (Hb < 8 g/dL) (n = 31) . | Partial response (Hb 8 to <11 g/dL) (n = 110) . | Complete response (Hb ≥ 11) (n = 7) . | ||
| Presplenectomy hemoglobin <7 g/dL† | 9/16 (56%)‡ | 12/54‡ (22%) | 0/2‡ | .007 |
| Age at splenectomy, median (range) | 3.7 (0.6-37.8) n = 31 | 4.1 (0.4-32.2) n = 110 | 11.7 (1.6-18.6) n = 7 | .3 |
| Total bilirubin, mg/dL, median (range) | 4.6 (1.1-17.6) n = 27‡ | 3.7 (1.0-14.3) n = 92‡ | 2.0 (1.1-3.1) n = 6‡ | .005 |
| Absolute reticulocyte count (× 106 cells/μL), median (range) | 0.8 (0.1-6.5) n = 13‡ | 0.7 (0.2-5.4) n = 28‡ | 0.6 (0.5-0.6) n = 3‡ | .16 |
| Reticulocyte count (%) median (range) | 33.9 (2.5-61.3) n = 20‡ | 27.1 (4.3-69.3) n = 85‡ | 16.9 (13.6-30.5) n = 5‡ | .11 |
| Currently receiving regular transfusions | 6/30 (20%) | 13/108 (12%) | 0/6 (0%) | .15 |
| Amish | 0/31 (0%) | 50/110 (46%) | 1/7 (14%) | .001 |
| Genotype§ | ||||
| M/M | 9/31 (29%) | 34/58 (59%) | 6/6 (100%) | .0017 |
| M/N | 10/31 (32%) | 15/58 (26%) | 0/6 (0%) | .0005 |
| N/N | 12/31 (39%) | 9/58 (16%) | 0/6 (0%) | .5|| |
| . | Response to splenectomy . | P* . | ||
|---|---|---|---|---|
| No response (Hb < 8 g/dL) (n = 31) . | Partial response (Hb 8 to <11 g/dL) (n = 110) . | Complete response (Hb ≥ 11) (n = 7) . | ||
| Presplenectomy hemoglobin <7 g/dL† | 9/16 (56%)‡ | 12/54‡ (22%) | 0/2‡ | .007 |
| Age at splenectomy, median (range) | 3.7 (0.6-37.8) n = 31 | 4.1 (0.4-32.2) n = 110 | 11.7 (1.6-18.6) n = 7 | .3 |
| Total bilirubin, mg/dL, median (range) | 4.6 (1.1-17.6) n = 27‡ | 3.7 (1.0-14.3) n = 92‡ | 2.0 (1.1-3.1) n = 6‡ | .005 |
| Absolute reticulocyte count (× 106 cells/μL), median (range) | 0.8 (0.1-6.5) n = 13‡ | 0.7 (0.2-5.4) n = 28‡ | 0.6 (0.5-0.6) n = 3‡ | .16 |
| Reticulocyte count (%) median (range) | 33.9 (2.5-61.3) n = 20‡ | 27.1 (4.3-69.3) n = 85‡ | 16.9 (13.6-30.5) n = 5‡ | .11 |
| Currently receiving regular transfusions | 6/30 (20%) | 13/108 (12%) | 0/6 (0%) | .15 |
| Amish | 0/31 (0%) | 50/110 (46%) | 1/7 (14%) | .001 |
| Genotype§ | ||||
| M/M | 9/31 (29%) | 34/58 (59%) | 6/6 (100%) | .0017 |
| M/N | 10/31 (32%) | 15/58 (26%) | 0/6 (0%) | .0005 |
| N/N | 12/31 (39%) | 9/58 (16%) | 0/6 (0%) | .5|| |
The population for this table is 148 patients who were splenectomized and had known data for response to splenectomy.
Hb, hemoglobin.
P value from ordinal logistic regression model.
Median presplenectomy hemoglobin was 6.7 g/dL (range, 5-12.5 g/dL) for the no-response group, 7.5 g/dL (range, 4.5-13.5 g/dL) for the partial response group, and 8.5 g/dL (range, 7-10 g/dL) for the complete response group.
Data were available only for subsets of patients (some patients had unknown data).
Amish participants were excluded from the genotype categories.
In the ordinal logistic regression model of response to splenectomy, the NM/NM group was the reference group.
Age at the time of splenectomy. Overall, 150 (59%) of 254 patients had a history of splenectomy at enrollment with median age at splenectomy of 4.1 years (range, 0.4-37.8 years). Among the cohort of Amish patients, 93% had a splenectomy at a median age of 1.6 years (range, 0.6-28.2 years). Among the cohort of non-Amish patients, 50% had a splenectomy at a median age of 5.0 years (range, 0.4-37.8 years).
Age at the time of splenectomy. Overall, 150 (59%) of 254 patients had a history of splenectomy at enrollment with median age at splenectomy of 4.1 years (range, 0.4-37.8 years). Among the cohort of Amish patients, 93% had a splenectomy at a median age of 1.6 years (range, 0.6-28.2 years). Among the cohort of non-Amish patients, 50% had a splenectomy at a median age of 5.0 years (range, 0.4-37.8 years).
Seventy-five percent (98 of 131) of patients age 18 years and older at enrollment had a prior splenectomy compared with 42% (52 of 123) who were younger than age 18 years at enrollment (P < .0001). There was no significant difference in age at the time of the first transfusion between patients who had a splenectomy and those who did not (P = .76). Univariate predictors of a poor response to splenectomy, defined as a postsplenectomy hemoglobin <8 g/dL included lower presplenectomy hemoglobin (P = .007), NM/NM mutations (P = .0017), and a higher total bilirubin (P = .005; Table 2).
Three patients had a partial splenectomy. Two patients, each of whom had a partial splenectomy at age 4 years, had a hemoglobin level of 6.9 g/dL after partial splenectomy, and both eventually discontinued regular transfusions. The third patient had a partial splenectomy at age 11 years and has continued to receive regular transfusions for 4 years postsurgery.
Postsplenectomy sepsis occurred in 7% of patients. Despite the earlier median age of splenectomy in the Amish cohort, there was no difference in the rate of postsplenectomy sepsis compared with the non-Amish cohort.
Of the 254 patients, 7% (17 of 252) had a history of thrombosis. There was an increased frequency of thrombosis in patients who underwent splenectomy (17 [11%] of 149) vs no occurrences of thrombosis in patients who were not splenectomized (P = .0001). Postsplenectomy thrombosis included deep vein thrombosis (n = 9), pulmonary embolism (n = 6), stroke (n = 3), and portal vein thrombosis (n = 2). Five patients had more than 1 thrombotic event.
Cholecystectomy.
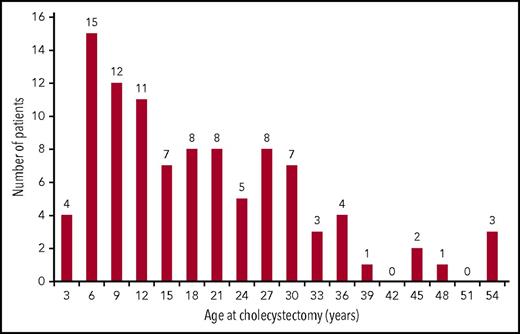
Gallstones are a frequent complication of PK deficiency and were reported in 45% (112 of 248) of patients with a median age of 14.3 years (range, 2.2-60.4 years; Figure 2), and 62% occurred in children younger than age 18 years. Cholecystectomy was performed in 101 (40%) of 254 patients. In patients who had a record of the timing of both procedures (n = 85), the cholecystectomy was performed together with splenectomy in 18 (21%) of 85 and after splenectomy in 56 (66%) of 85. Of the 121 patients who underwent splenectomy without a prior or simultaneous cholecystectomy, 58 (48%) required a cholecystectomy at a later time.
Age at the time of cholecystectomy. Cholecystectomy was performed in 101 (40%) of 254 participants overall and in 89 (79.5%) of 112 patients who reported gallstones. Median age at cholecystectomy was 15.1 years (range, 0.6 to 53.7 years).
Age at the time of cholecystectomy. Cholecystectomy was performed in 101 (40%) of 254 participants overall and in 89 (79.5%) of 112 patients who reported gallstones. Median age at cholecystectomy was 15.1 years (range, 0.6 to 53.7 years).
Comparing patients who required cholecystectomy with those who did not, there was no significant difference in hemoglobin (median, 8.8 vs 8.9 g/dL, respectively; P = .78). Patients who had a cholecystectomy had a significantly higher total bilirubin (median, 4.2 vs 3.4 mg/dL; P = .0024), higher absolute reticulocyte count (median, 0.5 vs 0.2 × 106 cells per μL; P = .0011) and older age at diagnosis (median, 0.7 vs 0.3 years, respectively; P = .04) than patients who did not have a cholecystectomy.
Spectrum of transfusion requirements
Of 250 patients with a known transfusion history, 210 (84%) had received a blood transfusion in their lifetime. Forty-eight percent of patients (25 of 52) age 5 years and younger received regular transfusions compared with 26% of patients (9 of 35) older than age 5 years to younger than 12 years, 11% (4 of 36) age 12 years to younger than 18 years, and 8% (10 of 127) age 18 years and older (P < .0001). Despite the transfusion frequency in this study cohort, only 3% (7 of 231) had allo-immunization, and 2% (5 of 224) had auto-immunization.
The most commonly reported hemolytic triggers for patients were infections (60%), stress (29%), and pregnancy (51%). Medications, including aspirin and/or anti-inflammatories (n = 6) and antibiotics (n = 3), were uncommon hemolytic triggers.
Patients age 5 years and younger at the time of enrollment.
Transfusion requirements are considered separately for patients age 5 years and younger (n = 52) and those older than age 5 years (n = 202), because splenectomy is usually not performed until after age 5 years. Among the patients age 5 years and younger, 6 (12%) had a splenectomy before enrollment, and 6 (12%) had never been transfused. Twenty-three patients (44%), all with intact spleens, were regularly transfused; median pretransfusion hemoglobin was 7.2 g/dL (range, 5.2-8.7 g/dL), median transfusion interval was 5.2 weeks (range, 2.7-8.7 weeks), and median volume of red cells transfused was 15.2 mL/kg.
Patients older than age 5 years at the time of enrollment.
Never transfused.
Of the 202 patients older than age 5 years at the time of enrollment, transfusion history was available for 198 (Table 3). A minority of these patients had never received a blood transfusion (34 [17%] of 198). These patients had a median enrollment age of 28 years (range, 5.9-69.9 years) and were observed at 11 different centers. This cohort had a median hemoglobin of 11 g/dL (range, 9-13.5 g/dL), and 20% (7 of 34) had previously undergone a splenectomy.
Transfusion status at the time of enrollment and hemolytic triggers
| . | Number of patients (n/N with known data) . | % . | Amish patients . | % . | Non-Amish patients . | % . |
|---|---|---|---|---|---|---|
| Transfusion status at enrollment* | ||||||
| Historically on regular transfusions† | 79/198 | 40 | 32/46 | 70 | 47/152 | 31 |
| Currently on regular transfusions‡ | 23§/198 | 12 | 0/46 | 0 | 23/152 | 15 |
| Intermittent transfusions only | 56/198 | 28 | 6/46 | 13 | 50/152 | 33 |
| Never transfused | 34/198 | 17 | 4/46 | 9 | 30/152 | 20 |
| Historical transfusions with unknown transfusion frequency | 6/198 | 3 | 4/46 | 9 | 2/152 | 1 |
| Hemolytic triggers | ||||||
| Presumed infections | 137/228|| | 60 | 41/53 | 30 | 96/175 | 70 |
| Stress | 59/207|| | 29 | 36/54 | 61 | 23/153 | 39 |
| Pregnancy | 20/39¶ | 51 | 13/41 | 33 | 26/89 | 67 |
| Transfused for an acute stressor | 127/247|| | 51 | 16/52 | 13 | 111/195 | 87 |
| Transfusions in the 12 mo prior to enrollment | ||||||
| Transfused at least once | 92/254 | 36 | 5/55 | 9 | 87/199 | 44 |
| Median hemoglobin (range) | 8.1 (5.5-11) | 8.8 (7.4-9) | 8 (5.5-11) | |||
| Not transfused | 162/254 | 64 | 50/55 | 91 | 112/199 | 56 |
| Median hemoglobin (range) | 9.6 (6.3-14.2) | 9.5 (8-11.2) | 9.6 (6.3-14.2) |
| . | Number of patients (n/N with known data) . | % . | Amish patients . | % . | Non-Amish patients . | % . |
|---|---|---|---|---|---|---|
| Transfusion status at enrollment* | ||||||
| Historically on regular transfusions† | 79/198 | 40 | 32/46 | 70 | 47/152 | 31 |
| Currently on regular transfusions‡ | 23§/198 | 12 | 0/46 | 0 | 23/152 | 15 |
| Intermittent transfusions only | 56/198 | 28 | 6/46 | 13 | 50/152 | 33 |
| Never transfused | 34/198 | 17 | 4/46 | 9 | 30/152 | 20 |
| Historical transfusions with unknown transfusion frequency | 6/198 | 3 | 4/46 | 9 | 2/152 | 1 |
| Hemolytic triggers | ||||||
| Presumed infections | 137/228|| | 60 | 41/53 | 30 | 96/175 | 70 |
| Stress | 59/207|| | 29 | 36/54 | 61 | 23/153 | 39 |
| Pregnancy | 20/39¶ | 51 | 13/41 | 33 | 26/89 | 67 |
| Transfused for an acute stressor | 127/247|| | 51 | 16/52 | 13 | 111/195 | 87 |
| Transfusions in the 12 mo prior to enrollment | ||||||
| Transfused at least once | 92/254 | 36 | 5/55 | 9 | 87/199 | 44 |
| Median hemoglobin (range) | 8.1 (5.5-11) | 8.8 (7.4-9) | 8 (5.5-11) | |||
| Not transfused | 162/254 | 64 | 50/55 | 91 | 112/199 | 56 |
| Median hemoglobin (range) | 9.6 (6.3-14.2) | 9.5 (8-11.2) | 9.6 (6.3-14.2) |
N, total number of patients with known data; n, number of patients.
The sample size is 202 because 53 children age 5 years and younger were not included in this analysis.
Historically received regular transfusions is defined as not receiving regular transfusions in the 12 months before enrollment and .6 transfusions per year before the 12-month period before enrollment.
Currently receiving regular transfusions is defined as receiving $6 transfusions over the last 12-month period before enrollment.
Twenty-three patients were receiving regular transfusions at the time of enrollment; 17 had had a splenectomy.
The denominators are less than 254 because some patients had unknown data for presumed infections, stress, or transfusion for an acute stressor.
Thirty-nine female patients had at least 1 pregnancy.
Intermittently transfused.
Approximately one quarter of patients older than age 5 years at enrollment (56 [28%] of 198) received intermittent transfusions but had never been regularly transfused. In this group, 61% (34 of 56) had previously been splenectomized.
Regular transfusions discontinued after splenectomy.
Most of the patients who historically required regular transfusions (≥6 transfusions over a 12-month period) no longer required regular transfusions after splenectomy. Of 79 patients who received regular transfusions up until the time of splenectomy, 62 (78%) were able to discontinue transfusions. In this group, the median hemoglobin was 8.8 g/dL (range, 4.3-12.8 g/dL) after splenectomy, and the median reticulocyte count was 32.3% (range, 4.8%-65%).
Regularly transfused after splenectomy.
Six patients older than age 5 years were regularly transfused and were not splenectomized. In addition, 17 patients (14% [17 of 124] of all patients splenectomized) continued to receive regular transfusions after splenectomy. The median number of weeks between transfusions in these patients was 5.8 (range, 2.4-8.7 weeks) with a median hemoglobin of 8.1 g/dL (range, 6.7-9.9 g/dL) before transfusion. The median volume of red cells transfused was 9.6 mL/kg. The median age at the time of splenectomy was 5.2 years (range, 2.1-25.8 years). The median reticulocyte count was 20.9% (range, 2.5%-44.1%) after splenectomy. Iron overload was more common in these patients (median ferritin, 1178 ng/dL [range, 307-3850 ng/mL]; 16 [94%] of 17 required chelation therapy) than in patients who discontinued regular transfusions after splenectomy (median ferritin, 624 ng/dL [range, 230-2783 ng/mL]; 28 [44%] of 62 required chelation therapy; P = .02 and P = .0002, respectively).
Laboratory findings.
Increased lactate dehydrogenase was not common (supplemental Table 2), whereas indirect hyperbilirubinemia was almost always present, with a median total bilirubin of 3.7 mg/dL. Those with a prior splenectomy had a higher reticulocyte count, mean cell volume, and total bilirubin. Median ferritin was 606 ng/mL (range, 17-8080 ng/mL [n = 180]) for the overall study cohort. Iron overload was common, even in those who did not receive regular transfusions. Forty-seven percent (87 of 184) of patients had a ferritin level of >1000 ng/mL or had received chelation therapy in the 12 months before enrollment. Ferritin level had not been checked in the year before enrollment in 29% (74 of 254) of patients.
Medications.
Commonly reported medications in the cohort included folic acid (182 [72%] of 252), iron chelation (83 [23%] of 254), and prophylactic antibiotics (49 [19%] of 253). Of those who had a splenectomy, 5% (12 of 150) were taking aspirin. Patients taking folic acid did not have a significantly different baseline hemoglobin (P = .4) or reticulocyte count (P = .8).
Genotype-phenotype associations.
The genotype-phenotype analysis was stratified as 112 patients with M/M mutations (58%), 52 with M/NM (27%), and 29 with NM/NM (15%; Table 4). For patients age 5 years and older at enrollment, all patients in the NM/NM group, 100% vs 62% in the M/NM group, and 56% in the M/M group had been splenectomized (P < .0001). Only 3% of the NM/NM group had never had a transfusion vs 18% of the M/NM and 23% of the M/M groups. There were significant differences in the total number of lifetime transfusions (P = .0015), with a median of 65 in the NM/NM group compared with medians of 25 in the M/NM group and 16 in M/M group. The NM/NM group had the highest maximum ferritin (P < .0001) and was more likely to have iron overload, defined as ferritin >1000 ng/mL or having received chelation in the year before enrollment (P = .0013). Notably, there was no evidence of an association of PK enzyme activity with the genotype.
Genotype-phenotype associations in 193 patients with pyruvate kinase deficiency
| . | NM/NM, N = 29, median (range) . | M/NM, N = 52, median (range) . | M/M, N = 112, median (range) . | P* . |
|---|---|---|---|---|
| Age at diagnosis, y | 0.4 (0-10.9) n = 29 | 0.7 (0-42.3) n = 50 | 1.3 (0-60.3) n = 106 | .049 |
| Hemoglobin, g/dL† | 7.9 (6.5-8.9) n = 14 | 8.4 (6.4-12.8) n = 21 | 8.9 (4.3-12.3) n = 41 | .006 |
| Total number of lifetime transfusions | 65 (3-363) n = 27 | 25 (1-516) n = 38 | 16 (1-486) n = 81 | .0015‡ |
| Maximum ferritin, ng/mL | 1787 (423-13409) n = 22 | 604 (22-8220) n = 37 | 573 (31-9679) n = 75 | <.0001‡ |
| Normalized PK enzyme activity, % | −41.6 (−152.4-15.2) n = 18 | −51.9 (−211.1-64.4) n=24 | −69.6 (−485.7-117.6) n = 60 | .16 |
| . | NM/NM, N = 29, median (range) . | M/NM, N = 52, median (range) . | M/M, N = 112, median (range) . | P* . |
|---|---|---|---|---|
| Age at diagnosis, y | 0.4 (0-10.9) n = 29 | 0.7 (0-42.3) n = 50 | 1.3 (0-60.3) n = 106 | .049 |
| Hemoglobin, g/dL† | 7.9 (6.5-8.9) n = 14 | 8.4 (6.4-12.8) n = 21 | 8.9 (4.3-12.3) n = 41 | .006 |
| Total number of lifetime transfusions | 65 (3-363) n = 27 | 25 (1-516) n = 38 | 16 (1-486) n = 81 | .0015‡ |
| Maximum ferritin, ng/mL | 1787 (423-13409) n = 22 | 604 (22-8220) n = 37 | 573 (31-9679) n = 75 | <.0001‡ |
| Normalized PK enzyme activity, % | −41.6 (−152.4-15.2) n = 18 | −51.9 (−211.1-64.4) n=24 | −69.6 (−485.7-117.6) n = 60 | .16 |
| . | NM/NM, n (%) . | M/NM, n (%) . | M/M, n (%) . | P§ . |
|---|---|---|---|---|
| Prenatal complications | 11/27 (41) | 15/48 (31) | 28/99 (28) | .44 |
| Exchange transfusion | 9/20 (45) | 17/41 (41) | 34/79 (43) | 1 |
| Rate of splenectomy in patients age ≥5 y | 21/21 (100) | 26/42 (62) | 48/88 (55) | <.0001 |
| Rate of cholecystectomy | 13/29 (45) | 25/52 (48) | 46/112 (41) | .69 |
| Extramedullary hematopoiesis | 3/25 (12) | 0/43 (0) | 5/93 (5) | .066 |
| Iron overload|| | 21/25 (84) | 20/38 (53) | 33/77 (43) | .0013‡ |
| Never transfused | 1/29 (3) | 9/51 (18) | 26/111 (23) | .036 |
| . | NM/NM, n (%) . | M/NM, n (%) . | M/M, n (%) . | P§ . |
|---|---|---|---|---|
| Prenatal complications | 11/27 (41) | 15/48 (31) | 28/99 (28) | .44 |
| Exchange transfusion | 9/20 (45) | 17/41 (41) | 34/79 (43) | 1 |
| Rate of splenectomy in patients age ≥5 y | 21/21 (100) | 26/42 (62) | 48/88 (55) | <.0001 |
| Rate of cholecystectomy | 13/29 (45) | 25/52 (48) | 46/112 (41) | .69 |
| Extramedullary hematopoiesis | 3/25 (12) | 0/43 (0) | 5/93 (5) | .066 |
| Iron overload|| | 21/25 (84) | 20/38 (53) | 33/77 (43) | .0013‡ |
| Never transfused | 1/29 (3) | 9/51 (18) | 26/111 (23) | .036 |
Amish patients with a homozygous R479H mutation were excluded from this analysis due to management differences, as these patients are typically uninsured and the cost of care can be prohibitive.
Kruskal-Wallis test.
Hemoglobin in the splenectomized, not regularly transfused cohort.
Statistically significant at P < .0055.
Fisher’s exact test.
Iron overload defined by maximum ferritin >1000 ng/mL, or the patient received chelation in prior 12 months.
Discussion
PK deficiency is a rare congenital hemolytic anemia with a diverse phenotype and wide spectrum of severity. A large international cohort of patients with PK deficiency is now followed in this multicenter, global natural history study, which allows for more detailed description of the clinical spectrum, complications, and current management strategies.
Most newborns present with significant jaundice, and about one quarter have had perinatal complications. The diagnosis of PK deficiency should be suspected in patients with perinatal jaundice and hemolysis. However, 10% of patients with PK deficiency in this study cohort did not have perinatal jaundice, so the absence of this feature does not exclude the possibility of PK deficiency. One patient presented with hepatosplenomegaly and hepatic dysfunction leading to hepatic failure, which has previously been reported by other groups.12,13 The evaluation of a newborn with noninfectious hepatic failure should include testing for PK deficiency.
Most patients with PK deficiency in this cohort received regular transfusions in early childhood. Young children experience more infectious stressors that lead to hemolytic episodes, and occasionally, young children will be transfused to support growth and development. However, the management of children age 5 years and younger is highly variable: some children have a splenectomy in the early years. The majority delay splenectomy beyond age 5 years but receive regular transfusions, others receive transfusions only for acute triggers, and some have never received a transfusion. This likely reflects both variability in clinical severity and differences in management strategies by hematologists because the hemoglobin nadirs overlap between transfusion groups. Although some newborns with PK deficiency will require transfusions after birth, decreasing the frequency of transfusions to permit a lower hemoglobin nadir will allow assessment of a reticulocyte response. The reticulocyte response may be adequate to transition to transfusion independence over time, even without a splenectomy. Because of the high levels of 2,3-diphosphoglycerate, a lower hemoglobin may be more tolerable and favorable in patients with PK deficiency compared with regular transfusions.14,15 The need for transfusions should be based on comprehensive clinical judgment regarding quality of life, growth, and symptoms and not on hemoglobin level alone.
In young patients who continue to rely on regular transfusions, the risk of transfusions must be balanced with the risks of splenectomy. On the basis of the data from this cohort, the risk of allo-sensitization is quite low, but transfusional hemosiderosis occurs in all patients, and regular transfusions have important implications for quality of life. The experience of the Amish cohort suggests that splenectomy at a younger age than is typical (younger than age 5 years) may avoid many years of iron loading in young patients who receive regular transfusions, but it comes with a potentially higher risk of serious bacterial infections. Of the 79 patients who were older than age 5 years at the time of enrollment and who received regular transfusions up until the time of splenectomy, 62 (78%) no longer received regular transfusions after splenectomy. Thus, splenectomy is effective for the majority of patients with PK deficiency, in that it allows patients to discontinue regular transfusions and improves anemia. The relationship between age and transfusions, in which older patients have a lower frequency of transfusions, is likely linked to the relationship between age and splenectomy and the effect of splenectomy on decreasing transfusion requirements. Anemia is only partially ameliorated by splenectomy, with a median hemoglobin of 8.8 g/dL after splenectomy, and 14% of those who underwent a splenectomy continued to require regular transfusions afterward.
Three patients in this cohort had partial splenectomies; one patient continued to be regularly transfused despite the procedure, and two others remained severely anemic after the procedure. These limited data are insufficient to answer the question fully, but based on our limited experience regarding partial splenectomy, full splenectomy seems to be the most beneficial for increasing hemoglobin levels and decreasing the need for transfusion. Although most patients no longer regularly received transfusions after splenectomy, those with a lower hemoglobin before splenectomy and more robust hemolysis were more likely to have a hemoglobin level of <8 g/dL after splenectomy. Thus, although patients with more severe hemolysis typically benefited from splenectomy in terms of transfusion needs, significant hemolysis predicts a less favorable hemoglobin response to splenectomy.
Splenectomized patients are at increased risk of venous thrombosis compared with the nonsplenectomized population.16 Patients who are splenectomized for hematologic conditions are at further increased risk of thrombosis compared with those splenectomized as a result of trauma. Specific congenital hemolytic anemias, such as hereditary xerocytosis, are associated with such a significantly increased rate of thrombosis after splenectomy, that changes in management have occurred, and splenectomy is often avoided in patients with these conditions.17 In this study cohort of 150 splenectomized patients with PK deficiency, 11% experienced postsplenectomy thrombosis, a rate similar to that described in other hematologic conditions.18-20 Although this important potential complication must be considered, the rates of thrombosis and pulmonary hypertension are not increased enough after splenectomy that splenectomy should be avoided in this patient population.
Gallstones are a frequent complication of PK deficiency at all ages from 2.2 to 60.4 years. Splenectomy does not decrease the risk of gallstones in patients with PK deficiency; thus, splenectomized patients with PK deficiency remain at risk for the development of pigmented gallstones. In this cohort, most patients who ultimately had both a cholecystectomy and a splenectomy had these surgeries performed separately. We conclude that just before splenectomy, all patients with PK deficiency should be screened for gallstones by ultrasound. If gallstones are present, concurrent cholecystectomy should be considered, because asymptomatic pigmented gallstones may lead to unnecessary complications. However, given the risk of complications from gallstones and a nearly 50% likelihood of a later cholecystectomy, cholecystectomy should be considered for all patients with PK deficiency at the time of splenectomy, regardless of the presence of gallstones by ultrasound.
The risk of transfusional hemosiderosis is a well-recognized, obligatory complication of regular transfusion therapy. Transfusion-independent iron loading in PK deficiency is under-recognized, occurring at all ages and in patients with both mild and more severe anemia. Approximately one quarter of the patients with PK deficiency in this cohort had not had ferritin levels checked in the year before enrollment. Because iron loading is common in PK deficiency, affected patients should have their iron levels monitored at least once per year. Patients with PK deficiency in this cohort also seemed to be at risk for bone disease and had bone deformities on physical examination along with a high rate of fractures. Bone disease is a common complication of thalassemia syndromes because of marrow expansion, iron toxicity and treatment, increased bone turnover, and hormone deficiencies.21 Patients with PK deficiency may be at similar risk for osteopenia; thus, close attention to vitamin D and calcium intake may be beneficial, and monitoring with dual-energy x-ray absorptiometry scans should be considered for baseline assessment in late adolescence or early adulthood.
A genotype-phenotype relationship was identified in this international cohort, in which patients with 2 M/M mutations in the PKLR gene had a lower likelihood of splenectomy, fewer lifetime transfusions, and a lower rate of iron overload. Patients with 2 NM/NM mutations were less likely to have a complete or partial response to splenectomy. Therefore, pursuing molecular testing may be useful in discussing prognosis and establishing a monitoring plan with patients on the basis of their genotyping results. Although a genotype-phenotype correlation was identified, there is a high frequency of complications in all genotypes. In addition, the ranges of hemoglobin levels were overlapping among the genotype groups, and so the genotype may not be predictive of clinical phenotype in some patients. Although genotyping in a young child can help predict the likelihood of complications, certain types of monitoring are needed in all patients.
The data from the Pyruvate Kinase Deficiency Natural History Study were limited by the nature of natural history studies and by patient registries shared among rare diseases. Despite the present global effort and large number of sites, a limited number of patients were available for data capture. Furthermore, deceased patients who may have had significant disease-related complications were not included, and only those with a laboratory diagnosis were included, thus excluding undiagnosed patients. In addition, patient or provider bias may have had an impact on historical data when medical records were not available. Furthermore, laboratory data, other assessments, and treatments such as splenectomy and transfusions were biased by provider practices, types of routine monitoring, and missing data.
The data from the Pyruvate Kinase Deficiency Natural History Study show few early clinical predictors of a later more severe clinical course. Serious complications including gallstones, iron overload, and osteopenia occurred even in those patients with nearly normal hemoglobin values, and serious but rare complications included pulmonary hypertension, extramedullary hematopoiesis, and lower extremity ulcerations in the cohort overall. Close monitoring and attention is needed in these patients, even in those with mild anemia. Despite these complications, life expectancy seems to be long. Together with the absence of predictors for disease severity, the potential for serious complications highlights the importance of close monitoring and follow-up of these patients by a hematologist, even for those patients with mild anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge all the patients with pyruvate kinase deficiency who contributed to this Natural History Study data and Anran Li for her assistance with statistical analysis.
The Pyruvate Kinase Deficiency Natural History Study was supported by research funding from Agios Pharmaceuticals.
Authorship
Contribution: R.F.G. conceived the study, analyzed and interpreted the data, and wrote the manuscript; W.B., A.Z., J.M.D., B.G., H.M.Y., S.W.E., E.J.v.B., M.S., M.M.M., P.B., and J.A.R. conceived the study, interpreted the data, and provided critical revisions to the manuscript; D.H.M., S.C., E.J.N., N.K., C.M.K., K.K., J.L.K., D.P., Y.D.P., P.E.N., Y.R., A.A.T., W.C.W., M.W.W., H.W., J.K., V.R.B., M.J.R., H.A.B., S.S., S.C., N.N., S.H., E.F., K.L.-G., and P.G.G. performed the research and contributed to the manuscript; and D.G., H.A.-S., and W.B.L. analyzed and interpreted the data and contributed to the manuscript.
Conflict-of-interest disclosure: R.F.G., P.B., H.M.Y., J.A.R., E.J.N., W.B., B.G., S.W.E., E.J.v.B., K.K., Y.R., S.C., and J.L.K. are scientific advisers to Agios Pharmaceuticals. R.F.G., E.J.v.B., B.G., H.M.Y., D.H.M., K.K., J.L.K., Y.R., S.S., W.B., and P.G.G. receive research funding from Agios Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Rachael F. Grace, Dana-Farber/Boston Children’s Cancer and Blood Disorder Center, 450 Brookline Ave, D3-106, Boston, MA 02215; e-mail: rachael.grace@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal