Key Points
Anti-CD43– and anti-CD44–antibody coating immobilizes live mouse and human HSPCs.
This enables 2D colony formation, medium exchange without cell-identification loss, and increased throughput of time-lapse imaging.
Abstract
Keeping track of individual cell identifications is imperative to the study of dynamic single-cell behavior over time. Highly motile hematopoietic stem and progenitor cells (HSPCs) migrate quickly and do not adhere, and thus must be imaged very frequently to keep cell identifications. Even worse, they are also flushed away during medium exchange. To overcome these limitations, we tested antibody coating for reducing HSPC motility in vitro. Anti-CD43– and anti-CD44–antibody coating reduced the cell motility of mouse and human HSPCs in a concentration-dependent manner. This enables 2-dimensional (2D) colony formation without cell mixing in liquid cultures, massively increases time-lapse imaging throughput, and also maintains cell positions during media exchange. Anti-CD43 but not anti-CD44 coating reduces mouse HSPC proliferation with increasing concentrations. No relevant effects on cell survival or myeloid and megakaryocyte differentiation of hematopoietic stem cells and multipotent progenitors 1-5 were detected. Human umbilical cord hematopoietic CD34+ cell survival, proliferation, and differentiation were not affected by either coating. This approach both massively simplifies and accelerates continuous analysis of suspension cells, and enables the study of their behavior in dynamic rather than static culture conditions over time.
Introduction
Maintaining single-cell identification over time is a prerequisite to understanding dynamic and heterogeneous cell behaviors and is lost by snapshot analysis of bulk or single-cell cultures.1,2 Continuous, quantitative time-lapse imaging has emerged as a powerful tool to answer long-standing questions and enables the investigation of dynamic single-cell behavior.3,4 Imaging frequencies need to be chosen carefully to ensure cell survival and identification while minimizing photo toxicity and frame-to-frame displacement. Adherent cells migrate slowly and proliferate in colonies; cell identities can be kept with low-imaging frequencies. Their adhesive properties prevent loss of cell identification during media exchange, making the study of dynamic single-cell responses during stimulation/starvation cycles or endpoint analysis after time-lapse observations feasible.5,6 In contrast, most hematopoietic cells are nonadherent or motile, and cell/clonal identities are lost unless images are acquired every minute, massively limiting the throughput of time-lapse studies. In addition, their cell identification is easily lost during media exchange. This poses a major challenge when studying dynamic single-cell responses of hematopoietic cells.
Classical hematopoietic assays retain clonal identity by plating cells in semisolid medium,7 stroma cocultures,8 or spatial separation in microwells,9 but do not provide continuous single-cell information. Although time-lapse imaging has been applied to these assays,10 technical drawbacks still hamper their application. Semisolid medium reduces cell motility, but induces 3-dimensional cell growth, requiring time consuming and more phototoxic image acquisition and analysis across z planes. Stromal cocultures only lead to minor motility reductions and are poorly defined. Single-cell deposition into microwells maintains clonal identity but does not reduce motility, and cell identifications are lost when cells divide.
We therefore tested whether surface-bound antibodies against surface antigens widely expressed on hematopoietic stem and progenitor cells (HSPCs)11 can reduce their motility, enabling lower imaging frequencies while keeping single-cell identification over time.
Study design
Mice
Experiments were conducted with 12- to 14-week-old male C57BL/6J mice from Janvier Labs according to Swiss federal law and ETH Zurich institutional guidelines, approved by local animal ethics committee Basel-Stadt (approval number 2655).
Hematopoietic cell isolation and culture
HSPCs were isolated12 and cultured as described13 using Iscove modified Dulbecco medium supplemented with 20% bovine serum albumin, insulin, and transferrin (BIT; Stemcell Technologies), 20 ng/mL CD41-phycoerythrin (MWReg30; eBioscience), 5 ng/mL CD16/32-BV421 (93; Biolegend), Annexin V Alexa Fluor 647 (Life Technologies) 1:5000.
Human CD34+ cell isolation
Time-lapse imaging
Statistical analyses
Unless stated differently data were analyzed using 2-way analysis of variance and corrected for multiple comparison. Mean ± standard error of the mean (SEM) are displayed using GraphPad Prism. Significant differences: *P < .05, **P < .01, ***P < .001.
Results and discussion
Anti-CD43 and anti-CD44 coatings reduce mouse HSPCs and human CD34+ CB motility and minimize cell loss during media exchange
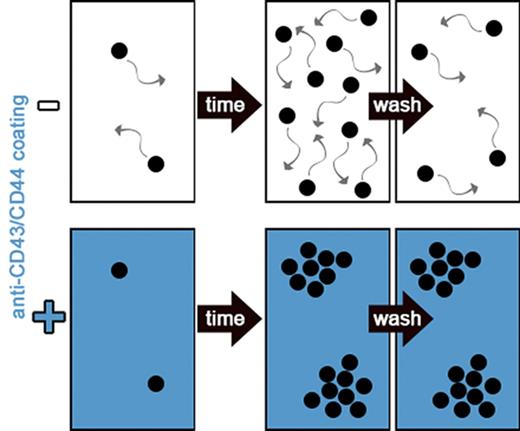
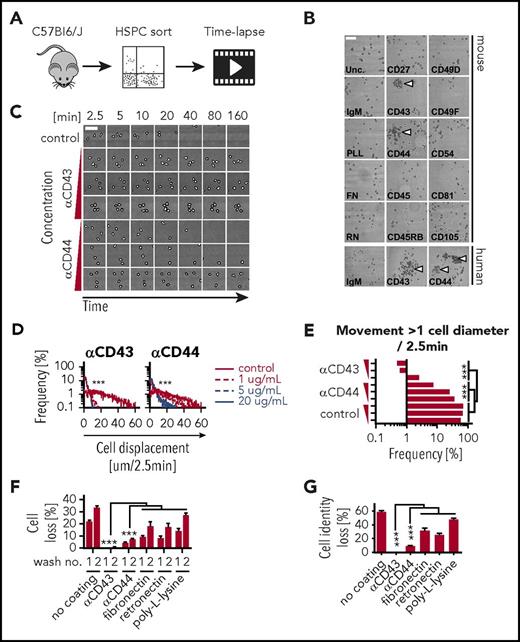
The high motility of HSPCs is a major obstacle in continuous single-cell analyses. We hypothesized that surfaces coated with antibodies against widely expressed cell-surface antigens would reduce HSPC motility. We cultured mouse Sca1+c-KIT+Lineage− (KSL) cells on anti-CD27, anti-CD43, anti-CD44, anti-CD45, anti-CD45RB, anti-CD49D, anti-CD49F, anti-CD54, anti-CD81, anti-CD105, poly-l-lysine, fibronectin, or retronectin coated surfaces (Figure 1A). Although cells were scattered uniformly on most coatings, colony formation was observed on anti-CD43 and anti-CD44 (Figure 1B). Dose-titration experiments showed that cell motility was reduced with increasing coating concentrations (Figure 1C) and that anti-CD43 was more potent than anti-CD44 (Figure 1D). At 5 μg/mL, anti-CD43 coating was sufficient to reduce the number of cells moving >1 cell diameter within 2.5 minutes to <0.7% (Figure 1E), which makes it possible to retain cell identifications at imaging intervals of 20 to 40 minutes (Figure 1C) as opposed to the currently required 60 to 90 seconds. This up to 40-fold reduction in imaging frequency allows more XY positions to be acquired per acquisition cycle, thus massively increasing the throughput of time-lapse experiments.
Anti-CD43 and anti-CD44 coatings reduce HSPC motility and enable medium exchange without cell-identification loss. (A) Freshly isolated KSL were sorted and incubated in 100 ng/mL stem cell factor (SCF), 100 ng/mL thrombopoietin (TPO), 10 ng/mL interleukin-3 (IL-3), 2 U/mL erythropoietin (EPO), and 10% fetal calf serum (FCS) on coated slides at indicated antibody or reagent concentrations and imaged every 2.5 minutes. (B) Representative images of KSL and human CD34+ CB on coated slides after 3 days. Colony formation (arrowheads) can only be recognized due to cell immobilization on anti-CD43 and anti-CD44 coating. In other conditions, forming colonies scatter and mix due to cell displacement. Scale bar, 100 μm. (C) Representative video frames of fixed region of interest (ROI) with increasing time intervals. Cells on uncoated slides are highly motile preventing reliable assignment of cell identifications (movement >1 cell diameter) over time and leave the ROI after about 40 minutes. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL. Scale bar, 50 μm. (D) Quantification of cell motility in micrometers per 2.5 minutes. Anti-CD43 reduces cell motility stronger and at lower concentration than anti-CD44 coating. n = 1 with 42-73 cells and 2124 to 4455 time points per condition analyzed. (E) Frequency of cell movements >1 cell diameter; 5 μg/mL anti-CD43–coating concentration is sufficient to keep single-cell identifications with high confidence. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL. (F-G) Cell and cell-identification loss (movement >1 cell diameter between 2 measurements) after medium exchanges. Virtually no or minor cell losses on slides coated with 20 μg/mL anti-CD43 and anti-CD44. Fibronectin, retronectin, and poly-l-lysine were used at 50 μg/mL, 100 μg/mL, 0.01% (wt/vol), respectively. n = 3 independent experiments, 260 to 298 and >62 to 306 cells per condition, respectively; mean ± SEM; χ2 test. FN, fibronectin; IgM, immunoglobulin M; PLL, poly-l-lysine; RN, retronectin; Unc., uncoated.
Anti-CD43 and anti-CD44 coatings reduce HSPC motility and enable medium exchange without cell-identification loss. (A) Freshly isolated KSL were sorted and incubated in 100 ng/mL stem cell factor (SCF), 100 ng/mL thrombopoietin (TPO), 10 ng/mL interleukin-3 (IL-3), 2 U/mL erythropoietin (EPO), and 10% fetal calf serum (FCS) on coated slides at indicated antibody or reagent concentrations and imaged every 2.5 minutes. (B) Representative images of KSL and human CD34+ CB on coated slides after 3 days. Colony formation (arrowheads) can only be recognized due to cell immobilization on anti-CD43 and anti-CD44 coating. In other conditions, forming colonies scatter and mix due to cell displacement. Scale bar, 100 μm. (C) Representative video frames of fixed region of interest (ROI) with increasing time intervals. Cells on uncoated slides are highly motile preventing reliable assignment of cell identifications (movement >1 cell diameter) over time and leave the ROI after about 40 minutes. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL. Scale bar, 50 μm. (D) Quantification of cell motility in micrometers per 2.5 minutes. Anti-CD43 reduces cell motility stronger and at lower concentration than anti-CD44 coating. n = 1 with 42-73 cells and 2124 to 4455 time points per condition analyzed. (E) Frequency of cell movements >1 cell diameter; 5 μg/mL anti-CD43–coating concentration is sufficient to keep single-cell identifications with high confidence. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL. (F-G) Cell and cell-identification loss (movement >1 cell diameter between 2 measurements) after medium exchanges. Virtually no or minor cell losses on slides coated with 20 μg/mL anti-CD43 and anti-CD44. Fibronectin, retronectin, and poly-l-lysine were used at 50 μg/mL, 100 μg/mL, 0.01% (wt/vol), respectively. n = 3 independent experiments, 260 to 298 and >62 to 306 cells per condition, respectively; mean ± SEM; χ2 test. FN, fibronectin; IgM, immunoglobulin M; PLL, poly-l-lysine; RN, retronectin; Unc., uncoated.
To determine whether the anti-CD43– and anti-CD44–mediated adhesion is also sufficient to reduce loss of cell identification during media exchange, we cultured KSL on anti-CD43, anti-CD44, poly-l-lysine, fibronectin, or retronectin, and quantified the number of lost cells (Figure 1F) and lost cell identifications (Figure 1G) after media replacement. Twenty percent to 30% of cells and 30% to 50% of cell identifications were lost on poly-l-lysine, fibronectin, or retronectin coating after 2 media exchanges, whereas cell and cell-identification loss with anti-CD43 and anti-CD44 coating was <1% and <8%, respectively, demonstrating that these coatings can be used to minimize cell (identification) loss during media exchange and subsequent analyses including immunostaining.
Cell death and differentiation of mouse HSPCs and human CD34+ CB on anti-CD43 and anti-CD44 coating
To further characterize the effects of reduction in cell motility in subpopulations, hematopoietic stem cells (HSCs), multipotent progenitors 1-5 (MPP1-5),20 pre–granulocyte/macrophage progenitors (pre-GMPs), GMPs, and pre–megakaryocyte/erythrocyte progenitors (pre-MegEs)21 were sorted (supplemental Figure 1, available on the Blood Web site) and cultured on anti-CD43 or anti-CD44. Colony formation and cell-motility reduction were observed in all populations (not shown) as expected, owing to the broad expression of both antigens.11 Next, we examined whether the coating affects survival by culturing the isolated populations with anti–Annexin V antibody to discriminate between living and apoptotic or dead cells during live imaging. No effect was detected within 24 hours except for MPP1s and GMPs on anti-CD43 coating (Figure 2A). However, with longer culture periods, increased cell death in GMP, pre-GMP, and pre-MegE was detected with anti-CD43 but not anti-CD44 coating. HSC and MPP1-5 survival remained unaffected on either coating (supplemental Figure 2). In addition, proliferation rates of all analyzed populations were unaffected by anti-CD44 coating, whereas anti-CD43 showed a concentration-dependent reduction in proliferation across all populations (Figure 2B). Lastly, to determine whether differentiation is affected by anti-CD43 and anti-CD44 coating, we cultured all subpopulations with “in culture” antibodies against CD16/32 and CD41, and quantified the myeloid and MegE lineage differentiation, respectively.4,17 Morphologically recognizable CD41+CD16/32− megakaryocytes and CD41−CD16/32+ macrophages were observed on both coatings; colonies with mixed, myeloid, or MegE potential were detected as in standard liquid-culture conditions9 (Figure 2C). Differentiation kinetics and frequencies of myeloid and MegE lineages are unaltered (Figure 2D; supplemental Figures 4-5).
Anti-CD43 and anti-CD44 coating exert minor effects on cell behavior. (A) Freshly isolated murine HSCs (KSL CD150+CD48−CD34−CD135), MPP1 (KSL CD150+CD48−CD34+CD135−), MPP2 (KSL CD150+CD48+CD34+CD135−), MPP3 (KSL CD150−CD48+CD34+CD135−), MPP4 (KSL CD150−CD48+CD34+CD135+), MPP5 (KSL CD150−CD48−), pre-GMP (KL CD41−CD16/32−CD150−CD105+), GMP (KL CD41−CD16/32+CD150−), and pre-MegE (KL CD41−CD16/32−CD150+CD105−) were sorted and incubated in 100 ng/mL SCF, 100 ng/mL TPO, 10 ng/mL IL-3, and 2 U/mL EPO, 10% FCS on coated slides at indicated antibody or reagent concentration and imaged every hour. Cell death was measured continuously by cell segmentation and quantification of Annexin V “in culture” antibody staining for 60 hours. Quantification of cell survival (frequency of Annexin V− cells) at 24 hours after culture start is displayed. Anti-CD43 and anti-CD44 coating had no or minor effects on cell survival across multiple HSPC populations. Triangles indicate increasing coating concentration range: 0, 10, and 20 μg/mL. n = 3 independent experiments, 83 to 252 cells per conditions, >2.7 × 106 quantified data points across 600 time points; mean ± SEM. (B) Proliferation is reduced only on high anti-CD43 concentrations, but not on anti-CD44 coating. n = 3 independent experiments, 41 to 461 cells per condition, >20.5 × 106 quantified data points (cells) across 160 measured time points total mean. SEM not shown for better readability. (C) Mixed, MegE, and myeloid colonies can be identified based on CD41 and CD16/32 “in culture” antibody staining. Scale bar, 100 μm. (D) Differentiation into myeloid (top) and MegE (bottom) lineage (measured by quantification of CD41 and CD16/32 “in culture” antibody staining after cell segmentation at day 6 of culture) is not affected by coating. n = 3 independent experiments, 41 to 461 cells per condition, >20.5 × 106 quantified data points (cells) across 160 measured time points total. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL; mean ± SEM. (E) Human CD34+ CB cells were cultured in Iscove modified Dulbecco medium supplemented with 20% BIT, 100 ng/mL SCF, 100 ng/mL Flt3L, and 50 ng/mL TPO. Cell death was measured continuously by cell segmentation and quantification of Annexin V “in culture” antibody staining for 5 days. Effects on survival 24 hours after culture are displayed. Anti-CD43 and anti-CD44 coating do not affect survival. n = 4 independent experiments, 1144 to 1757 cells per condition, >3.9 × 106 quantified data points (cells) across 320 measured time points total. Triangles indicate increasing coating concentrations: 0, 1, 5, and 20 μg/mL; mean ± SEM. (F) Proliferation of CD34+ CB (measured as number AnnexinV− cells over time) for 5 days. Anti-CD43 and anti-CD44 do not affect proliferation. n = 4 independent experiments; 1951 to 3039 cells per condition, >18 × 106 quantified data points (cells) across 320 measured time points total, mean. SEM not shown for better readability (G) CD34+ CB in vitro differentiation was measured by continuous quantification of CD34 “in culture” antibody staining for 5 days. Differentiation (measured by CD34 downregulation) is not affected by anti-CD43 or anti-CD44 coating. n = 2 independent experiments, 1358 to 2342 cells per condition, >16 × 106 quantified data points (cells) across 320 measured time points total, mean, SEM not shown for better readability. Conc., concentration; n.a., not available; n.s., not significant.
Anti-CD43 and anti-CD44 coating exert minor effects on cell behavior. (A) Freshly isolated murine HSCs (KSL CD150+CD48−CD34−CD135), MPP1 (KSL CD150+CD48−CD34+CD135−), MPP2 (KSL CD150+CD48+CD34+CD135−), MPP3 (KSL CD150−CD48+CD34+CD135−), MPP4 (KSL CD150−CD48+CD34+CD135+), MPP5 (KSL CD150−CD48−), pre-GMP (KL CD41−CD16/32−CD150−CD105+), GMP (KL CD41−CD16/32+CD150−), and pre-MegE (KL CD41−CD16/32−CD150+CD105−) were sorted and incubated in 100 ng/mL SCF, 100 ng/mL TPO, 10 ng/mL IL-3, and 2 U/mL EPO, 10% FCS on coated slides at indicated antibody or reagent concentration and imaged every hour. Cell death was measured continuously by cell segmentation and quantification of Annexin V “in culture” antibody staining for 60 hours. Quantification of cell survival (frequency of Annexin V− cells) at 24 hours after culture start is displayed. Anti-CD43 and anti-CD44 coating had no or minor effects on cell survival across multiple HSPC populations. Triangles indicate increasing coating concentration range: 0, 10, and 20 μg/mL. n = 3 independent experiments, 83 to 252 cells per conditions, >2.7 × 106 quantified data points across 600 time points; mean ± SEM. (B) Proliferation is reduced only on high anti-CD43 concentrations, but not on anti-CD44 coating. n = 3 independent experiments, 41 to 461 cells per condition, >20.5 × 106 quantified data points (cells) across 160 measured time points total mean. SEM not shown for better readability. (C) Mixed, MegE, and myeloid colonies can be identified based on CD41 and CD16/32 “in culture” antibody staining. Scale bar, 100 μm. (D) Differentiation into myeloid (top) and MegE (bottom) lineage (measured by quantification of CD41 and CD16/32 “in culture” antibody staining after cell segmentation at day 6 of culture) is not affected by coating. n = 3 independent experiments, 41 to 461 cells per condition, >20.5 × 106 quantified data points (cells) across 160 measured time points total. Triangles indicate increasing coating concentration range: 1, 5, and 20 μg/mL; mean ± SEM. (E) Human CD34+ CB cells were cultured in Iscove modified Dulbecco medium supplemented with 20% BIT, 100 ng/mL SCF, 100 ng/mL Flt3L, and 50 ng/mL TPO. Cell death was measured continuously by cell segmentation and quantification of Annexin V “in culture” antibody staining for 5 days. Effects on survival 24 hours after culture are displayed. Anti-CD43 and anti-CD44 coating do not affect survival. n = 4 independent experiments, 1144 to 1757 cells per condition, >3.9 × 106 quantified data points (cells) across 320 measured time points total. Triangles indicate increasing coating concentrations: 0, 1, 5, and 20 μg/mL; mean ± SEM. (F) Proliferation of CD34+ CB (measured as number AnnexinV− cells over time) for 5 days. Anti-CD43 and anti-CD44 do not affect proliferation. n = 4 independent experiments; 1951 to 3039 cells per condition, >18 × 106 quantified data points (cells) across 320 measured time points total, mean. SEM not shown for better readability (G) CD34+ CB in vitro differentiation was measured by continuous quantification of CD34 “in culture” antibody staining for 5 days. Differentiation (measured by CD34 downregulation) is not affected by anti-CD43 or anti-CD44 coating. n = 2 independent experiments, 1358 to 2342 cells per condition, >16 × 106 quantified data points (cells) across 320 measured time points total, mean, SEM not shown for better readability. Conc., concentration; n.a., not available; n.s., not significant.
To determine whether motility of human hematopoietic cells can also be reduced, umbilical CB–derived CD34+ cells were cultured on 1, 5, and 20 μg/mL anti-CD43 and anti-CD44 (Figure 1B). Neither anti-CD43 nor anti-CD44 altered cell survival (Figure 2E) or proliferation (Figure 2F). Differentiation kinetics as determined by CD34 downregulation were not affected by either coating (Figure 2G).
In summary, we describe a simple method to reduce the motility of mouse and human HSPCs by the use of surface coating by antibodies. This enables a massive increase in the throughput of time-lapse studies, without affecting survival, proliferation, and differentiation of HSPCs at the right antibody concentrations. In addition, it allows medium exchange without cell-identification loss, enabling the combination of time-lapse imaging with endpoint single-cell analysis. This will allow improved studies of cell responses in dynamic rather than static culture conditions, and shed light on how signal changes over time influence cell-fate decisions during homeostasis and disease. Optimal results can be obtained by using up to 5 μg/mL anti-CD43 coating, which minimizes advertent effects on proliferation while still significantly reducing cell motility.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marie-Didiée Hussherr and Gieri Camenisch for technical support.
This work was supported by grants from the Swiss National Science Foundation (T.S.), and the Systems X StemSysMed grant (I.M. and T.S.). W.W. was supported by the Swiss Initiative in Systems Biology Transition postdoc fellowship.
Authorship
Contribution: D.L. planned and performed experiments, collected and analyzed data, and wrote the manuscript with T.S.; W.W. and A.H. performed experiments and reviewed the manuscript; P.E.B. and I.M. supplied human HSPCs; F.R. designed the transmitted/fluorescence light switch; and T.S. designed and supervised the study, and developed and maintained long-term bioimaging with D.L. and segmentation software with O.H.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timm Schroeder, Department of Biosystems Science and Engineering (D-BSSE), Eidgenössische Technische Hochschule (ETH) Zurich, Mattenstr 26, 4058 Basel, Switzerland; e-mail: timm.schroeder@bsse.ethz.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal