In this issue of Blood, Swords et al report on promising data from a phase 1b combination study of the hypomethylating agent 5-azacitidine and pevonedistat, an inhibitor of the NEDD8-activating enzyme.1
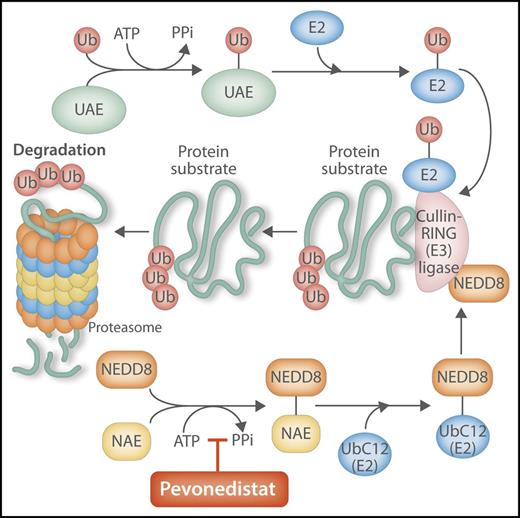
Pevonedistat inhibits the NEDD8-activating enzyme, with key effects on the ubiquitination pathway. Pevonedistat is a small-molecule inhibitor of NAE. NEDD8 is a ubiquitin-like protein that is modified and activated in similar fashion to “ubiquitination” by parallel and sequential enzymatic reactions, with both processes interacting and coordinating inside the cell to modify, shuttle, and degrade proteins through the proteasome. By inhibiting NAE, the normal activation in the ubiquitination process of the key ligase, CRL, is downregulated, interfering with the degradation of cellular targets by the proteasome, with resulting effects on cell cycling, apoptosis, and DNA replication. PPi, pyrophosphate; UAE, ubiquitin activation enzyme; Ub, ubiquitin; UBC12, NEDD8 conjugating enzyme. Professional illustration by Somersault 18:24.
Pevonedistat inhibits the NEDD8-activating enzyme, with key effects on the ubiquitination pathway. Pevonedistat is a small-molecule inhibitor of NAE. NEDD8 is a ubiquitin-like protein that is modified and activated in similar fashion to “ubiquitination” by parallel and sequential enzymatic reactions, with both processes interacting and coordinating inside the cell to modify, shuttle, and degrade proteins through the proteasome. By inhibiting NAE, the normal activation in the ubiquitination process of the key ligase, CRL, is downregulated, interfering with the degradation of cellular targets by the proteasome, with resulting effects on cell cycling, apoptosis, and DNA replication. PPi, pyrophosphate; UAE, ubiquitin activation enzyme; Ub, ubiquitin; UBC12, NEDD8 conjugating enzyme. Professional illustration by Somersault 18:24.
For decades, the conventional treatment of acute myeloid leukemia (AML) has been based on an aggressive 2-phased approach that seeks cure for patients but has often fallen short of that target. The goal of the first phase of therapy, intensive chemotherapy induction, is to achieve complete remission (CR), followed by a second phase, consolidation, which seeks to prolong remission and achieve cure. Consolidation can be additional cycles of intensive chemotherapy, but for many, including those with intermediate- or higher-risk disease, consolidative stem cell transplantation (SCT) is often pursued as a more aggressive curative attempt. Despite a relatively high proportion of patients achieving initial remission, refractory disease and frequent relapses are nevertheless a frequent feature of this conventional approach, and the proportion of patients with long term remissions and cure fall well below 50%. Outcomes are even poorer for older patients or those with high burden of comorbidity or functional limitations, for whom the aforementioned 2-step pradigm may not be an option due to its intensity and associated risk. For such cases, the emergence of hypomethylating agents (HMAs), 5-azacitidine or decitabine, has provided an emerging, well-tolerated therapeutic option.
However, HMAs are far from an optimal approach in terms of efficacy. Although many patients receiving these therapies have some degree of response, the rates of CR with 5-azacitdine or decitabine monotherapy are markedly lower than that seen with chemotherapy induction. Randomized phase 3 trials of HMA monotherapy in newly diagnosed AML patients have reported composite remission rates of 18% and 28% for decitabine and 5-azacitidine, respectively.2,3 Additionally, the achievement of any therapeutic response can take several months in some patients. Almost all who respond to HMAs ultimately experience disease progression, and in those who do respond, treatment duration is indefinite, requiring prolonged treatment courses and impact to quality of life. Because of their lower rate of associated remission, and because the patient populations receiving them are often older or functionally impacted, HMAs are infrequently followed by SCT and are not thought to be a curative paradigm.
In recent years, there have been multiple attempts to enhance the efficacy of HMAs for patients who are not candidates for intensive induction. These efforts have included extending the duration of therapy. Extending decitabine therapy to 10 days, as opposed to 5 days, appears to be well tolerated, and one phase 2 study yielded a very promising CR rate of 47%.4 More recently, 10-day decitabine was noted to be markedly efficacious in the high-risk cohort of patients with TP53-mutated AML.5 HMAs have also been combined with novel therapies in an effort to enhance overall efficacy but maintain tolerability. 5-Azacitidine and decitabine have been studied in combination with the antibody–drug conjugate vadastuximab talirine, as well as with the BCL2 inhibitor venetoclax. Both combinations, recently studied in smaller cohorts, have been associated with composite remission rates of >60%, leading to a degree of excitement.6,7 However, combining HMA therapies with other therapies raises concern for toxicity and decreased tolerability, important aspects for consideration as these combinations progress through clinical trial study and development.
In the current issue of Blood, Swords and colleagues report on another promising combination with HMA therapy for older patients with AML, one that incorporates pevonedistat, a small-molecule inhibitor of the NEDD8 (neural cell developmentally downregulated 8)–activating enzyme (NAE). Pevonedistat is thought to act through a novel anti-leukemic mechanism, by suppressing the activity of Cullin-RING E3 ubiquitin ligases (CRLs), which in turn play an integral, coordinated role in degrading key cellular targets mediated by the proteasome.8,9 When the activity of the CRLs are suppressed, substrates accumulate, impacting DNA replication and cell turnover and ultimately promoting antileukemic effects (see figure). Swords et al also point to preclinical data, including cell line studies and xenograft models, that suggested synergistic activity when 5-azactidine is combined with pevonedistat.
Monotherapy studies of pevonedistat in myeloid malignancies have reported modest rates of overall response, and doses of up to 83 mg/m2 were deemed tolerable.10 However, in the current combination study, the initial dose level studied (20 mg/m2) was also determined to be the maximum tolerated dose. Liver enzyme and bilirubin elevations were seen at a higher dose, and a small minority in the MTD expansion cohort also experienced grade ≥3 transaminase elevation. These events appeared to be transient and did not recur with subsequent lower-dose treatment. The range of adverse events seen on study otherwise seemed to reflect that frequently and typically seen with underlying AML and its treatment.
In addition to being well tolerated, this HMA combination also appears to be associated with promising rates of response. Among all 64 patients in the intention-to-treat cohort, the rate of overall response was 50%, with composite rates of remission being 39%. Additionally, the pace of response appeared to be relatively brisk, with >60% achieving their best response within 2 cycles of treatment. Although the numbers were small, it is encouraging that response rates were also particularly high among patients with TP53-mutated disease, a particularly high-risk group.
In summary, Swords and colleagues report on promising data for a novel HMA-based combination with the NEDD8-activating enzyme inhibitor pevonedistat. The treatment was well tolerated, with an encouraging overall response rate, which seems to have been achieved earlier and in a higher proportion of patients when compared with historical clinical trial data for HMA monotherapy. Additionally, the suggestion of activity in higher risk groups is exciting, and perhaps provides avenues for further studies focused on these populations. The authors also point to multiple upcoming combination studies, informed by preclinical data. The results of these and more advanced phase clinical study are necessary and eagerly awaited, with the hope that paradigms that incorporate and expand on HMA therapies will ultimately help improve outcomes for less robust patients with AML.
Conflict-of-interest disclosure: A.T.F. received consulting and advisory board fees from Takeda, Celgene, Seattle Genetics, Jazz, and Agios and has conducted clinical research supported by funding from Takeda, Celgene, and Seattle Genetics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal