Abstract
Background: It was once believed that African-American (AA) patients (pts) with multiple myeloma (MM) had more aggressive disease; however, recent studies suggest that undertreatment rather than biology is responsible for the poorer outcomes observed among AA pts. We recently reported that the utilization of stem cell transplants and bortezomib (BTZ) was 37% and 21% lower, respectively, among AA Medicare beneficiaries from 2000-2010 (Fiala & Wildes, Cancer, 2017). The data suggested that, had AA pts received these treatments at similar rates as their white counterparts there would have been no disparity in outcomes. As they did not, AA pts had a 10% increased risk for death. Lenalidomide (LEN), like BTZ, has become one of the primary treatments for MM since its approval in 2007. However, pts often incur significantly higher out of pocket costs on oral chemotherapies such as LEN compared to injectables like BTZ. Thus, we hypothesized that disparities in utilization of LEN would be greater than in BTZ.
Methods: To test our hypothesis, all MM cases from 2007-2013 in the SEER-Medicare were reviewed along with corresponding claims through 2014. We excluded cases with incomplete records including those not enrolled in Medicare Part A, B, and D, and HMO enrollees. We also excluded cases where MM was diagnosed prior to age 65 and suspected cases of smoldering MM, pts who did not receive MM treatment and did not have diagnostic codes for CRAB criteria as previously described (Fiala, et al, under review). The presence or absence of claims for LEN and BTZ within 6 months following MM diagnosis was used to determine utilization. Multivariate logistic regression was performed to analyze the impact of race on LEN and BTZ utilization controlling for age at diagnosis, sex, year of diagnosis, Medicaid enrollment, and previously established algorithms of poor performance status [PS] indicators and Charlson Comorbidity Index [CCI] score.
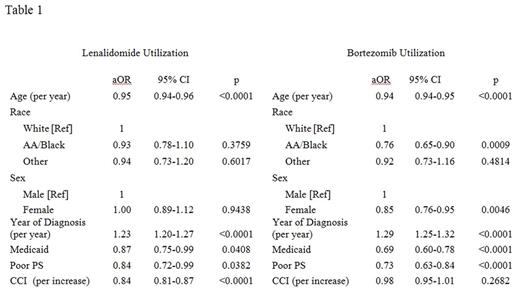
Results: 6,285 cases were included in the analysis. The median age at MM diagnosis was 76 years and 50% were female; 77% of cases were white, 16% were black, and 6% were another race. Overall, the utilization of LEN and BTZ during first-line treatment was 28% and 38%, respectively. In the multivariate model, race was not associated with LEN utilization, but BTZ utilization was reduced by 24% (aHR 0.76; 95% CI 0.65-0.90; p 0.0009) among AA pts. Overall, the models of LEN and BTZ were similar (Table 1). Advancing age, Medicaid enrollment and poor PS were associated with decreased utilization; more recent diagnosis increased utilization. CCI was associated with LEN but not BTZ utilization, sex with BTZ but not LEN.
Discussion: We originally hypothesized that LEN utilization would be reduced among AA pts based on increased out-of-pocket costs. However, we and other researchers have shown that traditional access barriers such as insurance and income only account for about 25% of health disparities (Fiala & Wildes, Cancer, 2017). We now hypothesize LEN's oral administration may mitigate some other factors that have been shown to increase disparities such as the preferences and biases of pts and providers. For example, AA pts may prefer oral therapies to a greater extent than white pts. Or AA pts who may decline injectable chemotherapy due to distrust in the medical system may be more willing to accept an oral formulation, or providers who hold an unconscious bias may be more willing to offer one.
An alternative explanation could be that AA pts, who tend to have more comorbidities and poorer overall health at MM presentation (Fiala, et al, 2015 ASH abstract 5618), were more likely to be candidates for LEN rather than other treatments. However, we do not believe this is the case as poor PS and increasing CCI were associated with decreased LEN utilization.
Conclusions: AA race was associated with reduced utilization of BTZ but not LEN for first-line treatment of MM. These findings do not support our original hypothesis that LEN is associated with a greater disparity in utilization than BTZ due to the higher cost sharing involved with oral chemotherapies. Whether oral formulations mitigate other barriers to treatment such as the preferences and biases among pts and providers requires future study.
Ailawadhi: Pharmacyclics: Research Funding; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Vij: Jazz: Honoraria; Konypharma: Honoraria; Celgene: Honoraria; Amgen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Abbvie: Honoraria; Janssen: Honoraria; Bristol-Meyers-Squibb: Honoraria. Wildes: Carevive Systems Inc: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal