Abstract
Introduction: The majority of young patients presenting with advanced-stage Hodgkin lymphoma (HL) in the UK are managed with ABVD. However, from 2009, escalated BEACOPP (escBEACOPP) was introduced as a treatment option in certain UK cancer centres. Within the East of England Cancer Network (EECN), 6 hospitals introduced escBEACOPP as a treatment option, while 2 hospitals continued to use ABVD as standard first-line therapy for all patients. During this time period, recruitment to the RATHL (Response Adjusted Therapy for Hodgkin Lymphoma) trial was also a first-line treatment option across the UK and 4 of the EECN centres. Analysing data from all patients treated in the EECN centres and the RATHL trial has therefore enabled outcomes of patients treated with either escBEACOPP or ABVD within a non-trial setting to be compared to patients treated with ABVD in a clinical trial.
Methods: We performed a retrospective multicentre analysis of patients treated for advanced-stage HL, aged 16-59 years, diagnosed between 2004-2014 in the EECN and compared outcomes with age and international prognostic score (IPS)-matched patients in the RATHL trial.
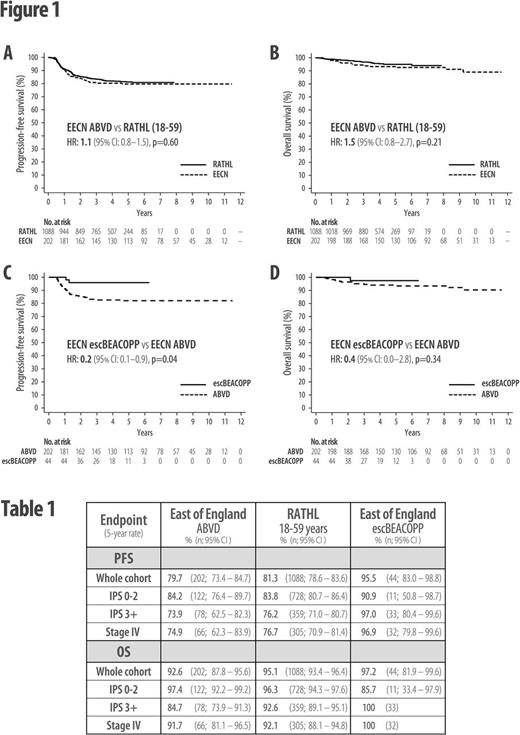
Results: Over this 10-year period, 250 patients were diagnosed with advanced-stage HL in the EECN; 202 commenced treatment with ABVD (23 within RATHL), 44 escBEACOPP, 3 other regimens, and 1 died before treatment. Five-year progression-free survival (PFS) for all patients was 82% and overall survival (OS) was 93%. In RATHL, 1088 patients aged 18-59 years commenced treatment with ABVD, with 5-year PFS of 81% and OS of 95%. Within the EECN, there was a clinician-patient preference to treat higher-risk patients (IPS 3+) with more intensive therapy; a higher proportion of escBEACOPP patients were IPS 3+ (IPS 3+: escBEACOPP 75% vs. ABVD 39%, p<0.0001).
ABVD-treated EECN patients had highly similar 5-year PFS and OS rates compared with age-matched RATHL patients (PFS 80% vs 81%; HR: 1.1 (95% CI: 0.8-1.5), p=0.60: OS 93% vs 95%; HR: 1.5 (95% CI: 0.8-2.7), p=0.21; Figure 1A-B). Despite being enriched with higher-risk patients, the 5-year PFS for all escBEACOPP-treated EECN patients was superior to ABVD in both the EECN and RATHL cohorts (PFS 95% vs 80% (EECN); HR 0.2 (95% CI: 0.1-0.9), p=0.04; Figure 1C; Table 1), but there was no significant OS advantage (5-year OS 97% vs. 93% (EECN); HR 0.4 (95% CI: 0.0-2.8), p=0.34; Figure 1D; Table 1). However, subgroup analysis of higher-risk (IPS 3+) patients found that escBEACOPP-treated EECN patients had both a 5-year PFS and OS advantage when compared with IPS 3+ ABVD-treated EECN patients (PFS 97% vs 74%; HR 0.1 (95% CI: 0.0-0.8), p=0.03; OS 100% vs 85%; p=0.03). However, when these higher-risk escBEACOPP patients were compared with IPS 3+ patients from RATHL, the significance of the OS advantage was lost (Table 1).
Despite similar relapse/refractory rates for IPS 3+ ABVD-treated patients in the EECN and RATHL datasets, the OS for relapsed/refractory patients was inferior in the EECN. One explanation could be the time period over which the EECN data was collected, as the majority (7/9) of higher-risk ABVD-treated EECN patients who died from progressive disease died prior to 2011, when brentuximab vedotin was unavailable to treat relapsed HL in the UK.
Conclusion: Our data support recently published European data showing that first-line HL patients treated in the non-trial setting achieve similar outcomes to those treated in contemporary clinical trials. Our data also reflect prospective trial results which show a first-remission PFS, but not OS, advantage for advanced-stage HL patients treated with escBEACOPP compared with ABVD, but suggest that higher-risk patients might benefit disproportionately from more intensive first-line therapy with escBEACOPP. However, improved access to more effective salvage treatments for relapsed/refractory patients may have diminished any OS benefit from the use of first-line escBEACOPP in higher-risk patients.
Follows: Abbvie: Other: advisory board and lecturing; Janssen: Other: advisory board and lecturing; Gilead: Other: advisory board and lecturing; Roche: Other: advisory board and lecturing.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal