Abstract
Introduction
Dysferlinopathies is a group of orphan neuromuscular diseases associated with impairment of mRNA and defect in dysferlin protein expression in skeletal muscle tissue due to mutations in DYSF (Dystrophy-associated fer-1-like) gene. This group of diseases is having autosomal recessive type of inheritance and the frequency of occurrence is 1/1,300-1/200,000 in different populations. There are three main clinical phenotypes associated with dysferlin gene mutations: Limb girdle muscular dystrophy type 2B (LGMD2B), Miyoshi myopathy (MM) and distal myopathy with anterior tibial onset (DMAT) (Yakovlev et. al. Human Gene Therapy 2016). Currently there is no treatment for these diseases. Hence, the development of new methods of treating this group of diseases remains an urgent task, in particular, with the use of gene therapy. According to the literature, CD14+ cells play a key role in the pathogenesis of human dysferlinopathies and are involved in inflammatory reactions and pathological angiogenesis.
Methods
In the present work, by using previously created recombinant adenovirus Ad5-Dysf (Starostina et. al. Medicine 2016) that encodes human cDNA dysferlin gene and enables the expression of the recombinant dysferlin protein, genetic modification of CD14 + monocytes was carried out. Therefore, the following aims were set:
1. Isolation of CD14+ cells from a patient with dysferlinopathy and a healthy donor.
2. Genetic modification of CD14+ monocytes.
3. Conduct western blot analysis of mRNA expression of recombinant dysferlin protein in modified cells after viral transduction. Evaluate the effectiveness of viral transduction by analyzing the expression of EGFP protein (adenovirus Ad5-EGFP) which was taken as a positive control by fluorescence microscopy.
Results
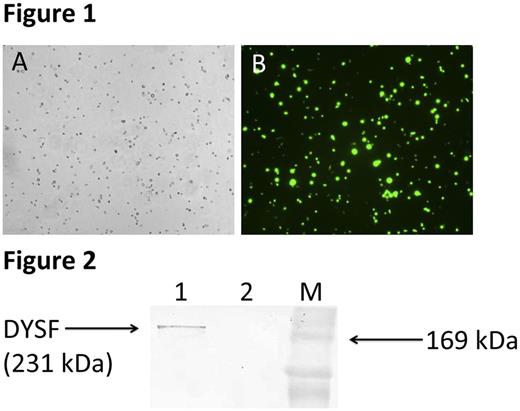
CD14+ cells were isolated from the freshly prepared peripheral blood leukocyte buffy coat layer of the patient with dysferlinopathy and a healthy donor by use of EasySep ™ Human Buffy Coat CD14 Positive Selection Kit. Since the correct assembly and effective DYSF gene delivery of the adenovirus Ad5-Dysf was confirmed in previous work, the viral transduction of human monocytes was carried out successfully. Ad5-EGFP adenovirus was used as a positive control to evaluate the effectiveness of viral transduction. EGFP protein expression analysis was conducted by fluorescence microscopy on an inverted fluorescent microscope of the research class AxioObserver.Z1 (Carl Zeiss) and demonstrated 100% of EGFP protein expression (Fig. 1). After genetic modification of the cells with resulting Ad5-Dysf adenovirus the evaluation of the recombinant dysferlin protein expression in cells was carried out by western blot analysis. Positive reaction was revealed with rabbit polyclonal antibodies to dysferlin (Fig. 2).
Conclusions
In this work we demonstrated that genetically modified CD14+ cells with DYSF overexpression can be successfully geberated and considered as one of the approaches for the therapy of human dysferlinopathies. Future studies are needed to analyze the functionality of the obtained genetic construct in animal models. SVV was supported by RFBR grant 16-34-00657.
Fig. 1. CD14+ monocytes genetically modified with Ad5-EGFP. A - phase-contrast microscopy. B - fluorescence microscopy. 48 hours after genetic modification. Magnification 200X.
Fig. 2. Western blot analysis of DYSF protein expression in CD14+ monocytes isolated from the peripheral blood of a patient with dysferlinopathy. Electrophoresis in 12% SDS-PAGE gel according to the Laemmli system. Antibodies to dysferlin (Abcam, ab15108) were used in a dilution of 1:250. The expected size of DYSF protein is 231 kDa. Well 1 - transduced CD14+ monocytes with Ad5-Dysf. Well 2 - native CD14+ monocytes. M - molecular weight marker.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal