Abstract
ProTmune, an ex vivo modulated mobilized peripheral blood (mPB) graft, has FDA fast track designation for the reduction of incidence and severity of acute graft-versus-host disease (GvHD) in patients undergoing allogeneic hematopoietic cell transplantation (HCT). Despite the use of standard protocols to prevent its occurrence, 50 to 60% of patients receiving an unrelated allogeneic HCT experience acute GvHD within 60 days post-HCT.
Here we report early Phase 1 safety and efficacy data from PROTECT, the ongoing Phase 1 open-label / Phase 2 blinded and randomized controlled trial (RCT) of ProTmune in adult subjects with hematologic malignancy undergoing HCT following myeloablative conditioning using a matched unrelated donor and receiving standard GvHD prophylaxis of methotrexate and tacrolimus.
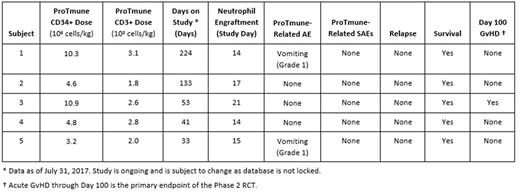
As of July 31, 2017, five subjects were administered ProTmune in the Phase 1 portion of PROTECT. Underlying hematologic diseases included ALL, AML and MDS. Subjects were predominantly female (80%), with mean age of 54 (range 34 to 66) and mean weight of 75.2 +/- 14.7 kg. All subjects remain on study as of July 31, 2017 with a median follow-up of 53 days (range 33 to 224 days).
ProTmune was manufactured on-site the day of HCT (Day 0) by pharmacologically modulating a mPB graft ex vivo with two small molecules (FT1050 and FT4145) to enhance the biologic properties and therapeutic function of the graft. All manufacturing runs have been successful, maintaining high cell viability (87.9 +/- 10.9%) and CD34+ cell recovery (87.7 +/- 13.5%). Successful pharmacologic modulation was confirmed by the marked increase in the percentage of cells positive for the potency marker CXCR4 (5.0 +/- 0.1% pre-manufacture to 67.3 +/- 7.9% post-manufacture). ProTmune, containing on average 6.8 +/- 3.6 x106 CD34+ cells/kg and 2.5 +/- 0.5 x108 CD3+ cells/kg, was administered on Day 0.
ProTmune has been well tolerated in these five subjects. ProTmune-related AEs were limited to two cases of Grade 1 vomiting on the day of administration. No ProTmune-related SAEs have been reported. All subjects engrafted with no reports of secondary graft failure. Median time to neutrophil engraftment was Day 15 (range Day 14 to Day 21). There have been no disease relapse or deaths.
The primary efficacy endpoint is incidence of acute GvHD through Day 100. In Phase 1, one subject was reported to have an acute GvHD event by Day 100 (limited to skin with complete recovery on methylprednisolone).
The Phase 1 portion of PROTECT is projected to enroll 6-10 subjects. By the 2017 ASH meeting, enrollment will be complete with at least six subjects projected to have reached Day 100+, and the safety and efficacy results will be updated.
Maziarz: Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria; Juno Therapeutics: Honoraria; Kite Therapeutics: Honoraria; Athersys, Inc: Patents & Royalties. Saad: Spectrum: Honoraria; Actinium: Consultancy. Diaz: Fate Therapeutics, Inc: Employment. Medcalf: Fate Therapeutics, Inc: Employment, Equity Ownership. Fremgen: Fate Therapeutics, Inc.: Employment. Storgard: Fate Therapeutics, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal