Abstract
Introduction
Ibrutinib (ibrut) and obinutuzumab (obin) are both highly active agents for patients (pts) with CLL, and are being combined in several ongoing clinical trials; however, the optimal order of administration of these two agents with respect to safety and efficacy remains undefined. We hypothesized that starting with ibrut prior to combining with obin would mitigate the risk of obin infusion reactions. We are conducting a 3-arm, randomized phase Ib study of ibrut plus obin in relapsed/refractory (R/R) CLL to determine the optimal order of administration.
Methods
The primary objective is to assess the safety of 3 dosing regimens of ibrut plus obin in pts with R/R CLL. Secondary objectives are to assess efficacy by ORR, CR, PFS, OS, and MRD status in the blood and marrow. Pts are randomized 1:1:1 to one of 3 cohorts, stratified by age ≥ or < 65 and by 17p status (Figure 1): Arm A starts with 1 cycle (4 wks) of obin prior to adding in ibrut 420 mg qd in cycle 2. Arm B starts with ibrut 420 mg qd for 4 wks prior to adding in obin. Arm C starts ibrut 420 mg qd and obin simultaneously. All pts receive the combination of ibrut with obin for 6 cycles (6 mo.), followed thereafter by ibrut maintenance until time of progression or unacceptable toxicity. Obin is administered on a standard schedule of 100 mg d1, 900 mg d2, 1,000 mg days 8, 15, and then 1,000 mg day 1 for 5 additional monthly cycles. To be eligible, pts must be R/R after at least 1 prior therapy, meet 2008 IW-CLL treatment criteria, have adequate organ function, and ECOG PS ≤2. Pts on prior ibrut or obin are excluded. IW-CLL 2008 and CTCAE v4 criteria are used to evaluate efficacy and toxicity, with response evaluations just prior to cycles 3, 7 (8 for arm B), and 13, and q6 mos. thereafter. MRD is assessed by 4-color flow cytometry (10-4 sensitivity).
Results
As of the data cutoff of July 5, 2017, 24 pts have been accrued, with 8 pts in each of the 3 arms. The median age at enrollment was 68 yrs (range 48-80). 19/24 pts (79%) were male. The median number of prior therapies was 1 (range 1-5). 4/24 (17%) had del(17p) and 5/24 (21%) had del(11q). Unmutated IGHV : 18/23 cases (78%), TP53 mutation: 9/23 (39%) tested, including 3 in each arm, and 1 pt in Arm A had a NOTCH1 mutation. Baseline β2M was 3.9 mg/L (range 0.7-15.1).
In an interim safety analysis, infusion reactions occurred in 5/8 (63%) pts in Arm A, including 4 Gr2 and a Gr3 reaction that required desensitization, which allowed successful re-challenge. No infusion reactions were observed in any of the 16 total pts on Arms B and Arm C. The number and severity of all grade non-hematologic toxicities in each arm was similar: Arm A: 7 (G1), 4 (Gr2), 1 (Gr3), Arm B: 7 (Gr1), 1 (Gr2), 2 (Gr3), Arm C: 7 (Gr1), 1 (Gr2), 4 (Gr3). All-grade non-hematologic toxicities occurring in ≥2 pts included: bruising, diarrhea, myalgias, extremity pain, and rash (n=2 each, all Gr1). Gr3 atrial fibrillation occurred in 1 pt on Arm C. Hematologic toxicity included: Arm A: 1 anemia (Gr1), and 1 each neutropenia and thrombocytopenia (both Gr 2), Arm B: 1 thrombocytopenia (Gr2), Arm C: 1 anemia (Gr1), 1 each neutropenia and thrombocytopenia (both Gr2), and 1 thrombocytopenia (Gr3). SAEs included Gr3 Bell's Palsy in 1 pt (on Arm B), Gr3 acute coronary syndrome in 1 pt (on Arm B), and Gr3 fungal pneumonia, cryptococcal meningitis, and pneumatosis intestinalis all in the same pt on Arm C who was recently refractory to a trial of a PI3K-inhibitor plus FCR. There were no fatal AEs.
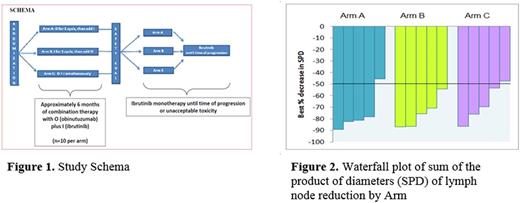
Pts have completed a median of 7.5 monthly cycles (range 1-19), with all pts still on treatment. In a preliminary analysis of the 21 pts evaluable for efficacy, the ORR was 90% (19/21), including 10% CR and 80% PR. ORR was similar in each arm: Arm A: 4/6 (67%) with 1 CR and 1 radiographic CR, Arm B: 7/7 (100%) with 1 radiographic CR, and Arm C: 6/8 (75%) with 1 CR. Depth of nodal response between the 3 arms was also similar in the pts with significant nodal disease (Figure 2). MRD analysis is in progress.
Conclusions
The rate of obin infusion reactions appears to be higher when obin is started prior to ibrut, compared to starting ibrut prior to obin or starting both drugs simultaneously. Other toxicities and efficacy are similar irrespective of order of administration. Accrual continues to this ongoing study (NCT02537613), and pts will be monitored for conversion to CR and MRD-negativity on ibrut maintenance. Our data may help define an evidence-based order of administration of this combination for future studies and eventually for clinical practice.
Davids: InCyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy; Celgene: Consultancy; Infinity: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Abramson: Gilead: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Abbvie: Consultancy; Kite Pharma: Consultancy; Seattle Genetics: Consultancy; Novartis: Consultancy; LAM Therapeutics: Research Funding. Jacobsen: Spectrum: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. LaCasce: BMS: Consultancy; Forty Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Research Funding. Fisher: Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Brown: Celgene: Consultancy; AbbVie: Consultancy, Honoraria; Astellas Pharma: Consultancy; AstraZeneca: Consultancy; Redx: Consultancy; Pfizer: Consultancy; Janssen: Consultancy; Janssen Oncology: Honoraria; Roche/Genentech: Consultancy; Infinity Pharmaceuticals: Consultancy; Pharmacyclics: Consultancy; Sun BioPharma: Consultancy, Research Funding; Gilead: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal