Abstract
Background: Much of the literature on anthracycline-associated cardiotoxicity has focused on late effects of exposure among childhood cancer survivors. Given the high anthracycline exposure in front-line therapy for pediatric acute myeloid leukemia (AML), it is crucial to gain a better understanding of the risk factors for early cardiotoxicity and its impact on subsequent treatment outcomes.
Methods: Between August 2006 and June 2010, Children's Oncology Group trial AAML0531 enrolled patients less than 30 years of age for frontline treatment of de novo AML. These analyses excluded Down syndrome patients. Cardiac events over the course of treatment and off-protocol follow-up were ascertained through a combination of passive (traditional adverse event (AE) case report forms) and active (targeted requests for echocardiograms for patients with an AE report for a cardiac event or life-threatening bloodstream infection) adverse event monitoring. The primary outcome of cardiotoxicity was defined as grade 2 or higher left ventricular systolic dysfunction (LVSD) based on Common Terminology Criteria for AE Version 3 definitions (i.e., ejection fraction <50% or fractional shortening <24%). We evaluated risk factors for incident LVSD using multivariable Cox regression analyses. Cox regression models were then used to compare event-free survival (EFS) and overall survival (OS) in patients with documented LVSD, classified as infection-associated and not infection-associated, relative to patients with no documented LVSD. The occurrence of LVSD was considered as a time-varying exposure to avoid immortal and immune person-time bias. Adjusted hazard ratios and corresponding 95% confidence intervals were reported. Cox model predicted survival probabilities were used to plot the OS and EFS curves.
Results: These analyses included 1022 AML patients enrolled AAML0531. Compliance with echocardiographic evaluations was higher but more variable during the on-protocol (median: 61.2%, range: 41.1%-85.0%) compared with the off-protocol reporting periods (median: 53.8%, range: 39.1%-56.1%). Compliance did not differ significantly by age, gender, race, ethnicity, BMI, insurance status, AML risk classification, initial white blood cell count, or randomized treatment arm.
Approximately 12% of patients had documented grade 2 or higher LVSD over the course of follow-up; 25% of incident events were classified as infection-associated. The cumulative incidence increased approximately linearly during on-protocol therapy from 1% to 8% from Induction I through Intensification III, but plateaued early during off-protocol follow-up (i.e.18 months). The overall incidence increased with age at enrollment (p-value for trend: <0.002), was higher among black compared to white patients (HR=1.73, 95% CI: 1.00, 2.99), among obese compared to normal weight patients (HR=1.64, 95% CI: 1.01, 2.65), and in the setting of a bloodstream infection (HR=1.86, 95% CI: 1.12, 10.9). These overall trends, with the exception of obesity, were driven by on-protocol toxicities.
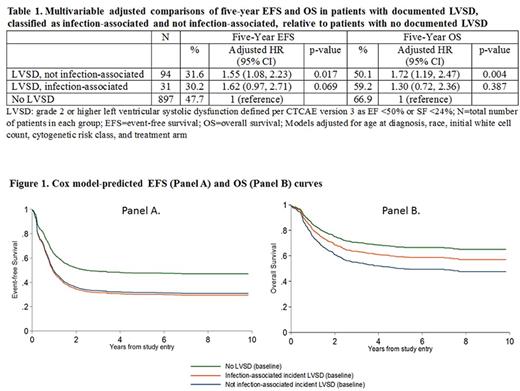
Five-year EFS and OS were significantly lower in patients who experienced on-protocol cardiotoxicity (Table 1, Figure 1). The impact on EFS was similar whether the incident event was determined to be in the presence or absence of infection (absolute differences of 17.5% and 16.1%, respectively), whereas the impact on OS was more pronounced for cardiac toxicity in the absence of infection than cardiac toxicity in the presence of infection (absolute differences of 16.8% and 7.7%, respectively).
Conclusions: Our findings demonstrate that early treatment-related cardiotoxicity is associated with decreased EFS and OS possibly through associated reductions in anthracycline exposure. The suggested heterogeneity in the effect on OS by infection status implies that patients who experience LVSD in the context of infection may be more salvageable upon relapse than patients experiencing non-infection related cardiotoxicity. Efforts to improve the outcomes for children with AML should include increased attention to the prevention and mitigation of on-protocol cardiac toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal