Abstract
Introduction:
Direct oral anticoagulants (DOACs) are indicated for stroke prevention in non-valvular atrial fibrillation (NVAF) and treatment of venous thromboembolism (VTE). Safety parameters, namely major bleeding and clinically relevant non-major bleeds (CRNMB) are shown to be lower in patients taking DOACs as compared to vitamin-K antagonists (VKAs). Several phase III trials performed subgroup analyses in obese patients, and found no difference in efficacy when compared to conventional VKAs or enoxaparin. Given global prevalence of obesity and paucity of safety data in this population, we aim to evaluate the safety and efficacy of therapeutic DOACs based on BMI (kg/m2).
Methods:
We performed a retrospective chart review of Medstar Washington Hospital Center EMR to include adult patients who were on apixaban, rivaroxaban or dabigatran for NVAF stroke prophylaxis or VTE in 2015. Patients were followed for 6 months in VTE group and up to 2 years for NVAF group. Patients were excluded if there was incomplete data. We categorized the patients based on WHO BMI classifications. Our primary safety outcome included major bleed and CRNMB. Efficacy was assessed in both NVAF and VTE patients as a secondary outcome.Statistical analysis was performed using Chi-square, fisher exact and logistic regression. A p-value <.05 was considered statistically significant.
Results:
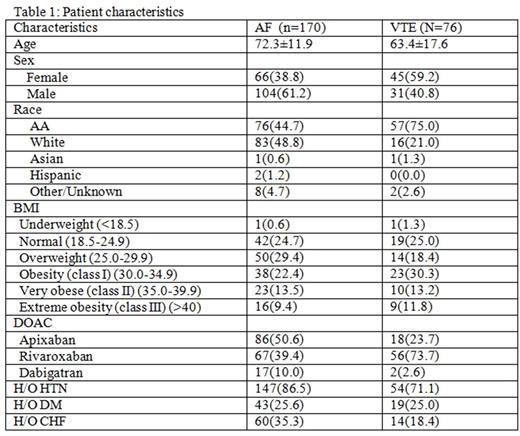
Of 246 patients, 170 had NVAF whereas 76 had VTE. Baseline characteristics are reported in Table 1. VTE patients were generally younger than the NVAF group and were more likely to be African American. 48% of the patients had a BMI >30mg/m2 and 10% were above 45mg/m2. Dabigatran numbers were too small for any statistical evaluation.
Nonvalvular Atrial fibrillation
The mean follow up time was 22.5±10.3 months. Slightly more major bleeding was noted in patients receiving apixaban (3.5%) than rivaroxaban (1.5%), but this was not statistically different across BMI groups. Eight patients had CRNMB in the apixaban group, which was not statistically significant across BMI groups. However, in patients taking rivaroxaban, there was a statistically significant relationship between BMI and CRNMB with a p-value 0.0004. In logistic regression analysis, BMI class I and class II predicted CRNMB with odds ratios of 0.08 (95% CI 0.008-0.781) and 0.097 (95% CI 0.010-0.971) respectively. This remains statistically significant when age adjusted. The association persists when all patients are combined (VTE and NVAF) with a p value of 0.04. There were only three stroke events, which were not significantly different across BMI groups.
VTE
After 6 months, there was a total five major bleeds (one in the apixaban group and four in rivaroxaban group). No patients in the apixaban group had CRNMB compared to 12.5% patients taking rivaroxaban. There was no statistically significant relationship found between BMI and either safety outcomes. VTE occurred in seven patients, however no relationship was found between either DOAC amongst BMI groups.
Discussion:
We present the first study specifically analyzing safety and efficacy of DOAC therapy based on BMI categories with good distribution across all groups. No differences were found across BMI groups, most importantly in BMI >40 kg/m2, further supporting previously published subgroup analyses of major clinical trials. Interestingly, we did find reduced CRNMB risk in patients with larger BMIs (Obese Class 1 and 2) taking rivaroxaban. Perhaps, this could be explained by lower peak concentrations and shortened half lives of DOACs in obese patients that have been suggested by some pharmacological studies. This difference was not seen in patients taking apixaban possibly due to a reduced dose (2.5mg) that is sometimes used in NVAF patients. Although this data supports that DOACs demonstrate similar efficacy and safety across BMI categories, prospective data is still needed to make more definitive conclusions about DOAC use in the obese population.
Fitzpatrick: Pfizer Honorarium: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal