Abstract
Hemophilia is an X-linked recessive bleeding disorder resulting from a mutation in the gene encoding clotting factors VIII (Hemophilia A) or IX (Hemophilia B). Although the current standard of care involves prophylactic factor VIII and IX replacement therapy, the need for multiple weekly intravenous infusions results in a high treatment burden, including problems with adherence and the frequent use of central venous access devices. The recent development of bioengineered coagulation factors with extended circulation times reduces the frequency of infusions and has the potential to improve the adherence to prophylactic treatment. Although clinical trials have demonstrated extended half-life (EHL) recombinant factor VIII and IX fusion (Fc) proteins to be safe and efficacious [Nolan et al., 2016; Shapiro et al., 2012], studies on real world clinical application have not been performed. As such, we chose to retrospectively examine the real-world experience of these two agents.
A retrospective review of existing medical records of patients with hemophilia A or hemophilia B who had been prescribed rFVIII Fc or rFIX Fc was conducted from the Children's Hospital Los Angeles Hemostasis and Thrombosis Center database.
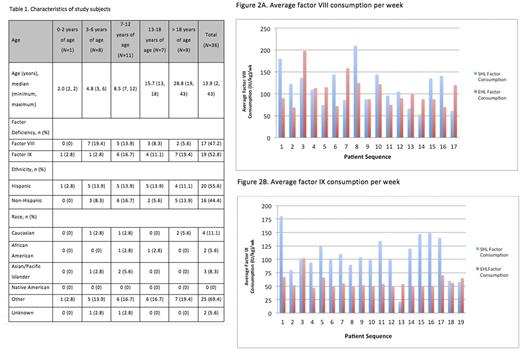
A total of 36 male subjects enrolled in the study (17 patients with hemophilia A and 19 patients with hemophilia B; 0-18 years of age, N= 27; > 18 years of age, N= 9). All 36 patients had been previously treated with standard half-life (SHL) recombinant FVIII (Advate, Helixate, rFVIII Fc as part of the A-Long Kids study [Young et al., 2015]) or recombinant FIX (Benefix) and on a prophylaxis regimen. Characteristics of the study subjects are specified in Table 1. Patients had a reduction of their annual bleed rate (ABR) and annual joint bleed rate (AJBR) following initiation of EHL factors. For patients with hemophilia A, the ABR and ABJR fell from 2.3 and 1.8 to 1.3 and 0.71, respectively. For patients with hemophilia B, the ABR and ABJR fell from 2.5 and 2.1 to 0.82 and 0.37, respectively.
In terms of factor consumption in patients with hemophilia A, EHL consumption level was lower than SHL consumption level for 9 of the 17 patients. Though the EHL consumption level appeared to be lower than the SHL consumption level, the difference was not statistically significant (p=0.45, paired t-test). The median factor consumption values for SHL and EHL were 110 and 90 (IU/kg)/wk, respectively. In patients with hemophilia B, EHL consumption level was lower than SHL consumption level for 16 of the 19 patients. A significant difference was found in SHL vs. EHL factor IX consumption level (p<0.001, paired t-test), with the EHL consumption level being significantly lower than the SHL consumption level. The median factor consumption values for SHL and EHL were 100 and 52 (IU/kg)/wk, respectively. Average factor consumption values for each hemophilia patient are displayed in Figures 2A and 2B.
The outcomes of clinical trials are not always recapitulated in the clinic. The results from this "real world" clinical experience, however, do support the results of the published clinical trials. First, the dosing frequency for both rFVIII Fc (2 days per week to every 5 days) and rFIX Fc (every 7 days to every 14 days) were comparable to those in the clinical trial [Mahlangu, Powell, & Pierce, 2014; Powell et al., 2013]. Second, we noted a low total ABR and AJBR consistent with the clinical trials for both rFVIII Fc and rFIX Fc, and importantly, patients on this study had a reduction of their ABR and AJBR following initiation of the EHL factors even though they had all been transitioned from a prophylaxis regimen [Mahlangu et al., 2014; Powell et al., 2013]. Third, it is noteworthy that patients who transitioned to an EHL FIX reduced their factor consumption by nearly half. While we did not conduct a formal pharmacoeconomic analysis, clearly the reduced factor consumption would at least offset to some extent the increased cost of the EHL factors [Shapiro et al., 2014]. In conclusion, this study demonstrates the largely successful transition of 36 patients from SHL to EHL factor products.
Young: CSL Behring: Honoraria; Novo Nordisk: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal