Abstract
Introduction
Carfilzomib (Kyprolis; K) is a selective 20S proteasome inhibitor indicated for the treatment of patients with relapsed or refractory multiple myeloma (MM). The indications for K in the US package insert (label) and dosing guidelines have been updated over time with the emergence of trial data. After July 25, 2015, K is to be dosed at 20mg/m2 for the first 2 doses and then dosed at 27mg/m2 or 56mg/m2 from the third dose in cycle 1 based on the ASPIRE and ENDEAVOR trials, respectively. Prior to the updates, K dose was recommended by label to increase from 20 to 27mg/m2 on the first day of the second K cycle. The purpose of this retrospective cohort study was to examine the extent to which K dosing in real-world settings is consistent with the label. Additionally, we compared time to next treatment (TTNT) between patients dosed per K label versus those treated with a lower K dose intensity.
Methods
The study population consisted of patients who received a K-based regimen during the course of treatment for MM between 11/01/2013 and 02/29/2016, were ≥18 years old, were not enrolled in a clinical trial, and had at least 2 visits to a US Oncology Network (USON) clinic. Data on K dosage was obtained from the USON electronic health records (EHR). Patients were categorized into two groups, 'K dose per label' or 'lower K dose intensity.' Definitions for dose per label were as follows for this analysis: 1. Patients starting treatment between 01 Nov 2013 - 25 July 2015: K dosed at 20 mg/m2 for the first cycle and escalated to 27 mg/m2 (±10%) on Day 1 in Cycle 2 or first escalation from 20 to 27 mg/ m2 between 25-30 days from first K dose; 2. 25 July 2015 - 21 January 2016: K dosed at 20 mg/m2 for the first two doses (Days 1 and 2 of Cycle 1) and escalated to 27 mg/m2 (±10%) by the third dose (Day 8 of Cycle 1); or 3. after 21 January 2016: escalated to 27 or 56 mg/m2 (±10%) by the third dose (Day 8 of Cycle 1). TTNT from K initiation was estimated with the Kaplan-Meier method, using log-rank tests for comparison, and was defined as the duration between first administration date of a K-based regimen and the first administration date of the next therapy. Patients who did not move onto a next treatment, were lost to follow up, or did not die during the study period were censored. A multivariable Cox regression model was built using stepwise selection (p for entry: 0.25; p for retention: 0.15). The variables available for inclusion in the stepwise model building process were baseline age, sex, race, geographic region, number of comorbidities, stage, body mass index (BMI), prior cancer history, performance status, serum creatinine, and per label K dosing status.
Results
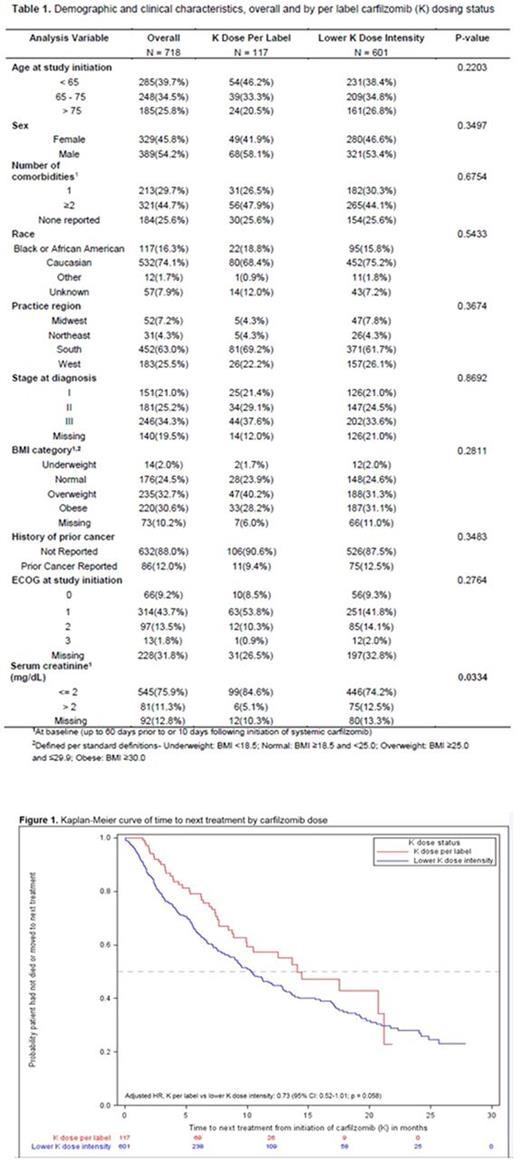
718 eligible MM patients who were treated with K at any point between their first and fifth lines of therapy were identified. A total of 117 (16.3%) patients were dosed per label during the study period, and 601 (83.7%) had a lower K dose intensity (Table 1). Baseline serum creatinine was significantly different between the two groups (p=0.03); patients dosed per label were less likely to have had an abnormal baseline serum creatinine. Median TTNT from K initiation was 10.6 months (95% CI: 9.4-13.0) for the study population. There was a significant difference in TTNT between patients treated with K who were dosed per label versus those with a lower dose intensity (Fig. 1; median TTNT for patients dosed per label: 14.1 months, 95% CI: 9.92-21.2; median TTNT for lower dose intensity patients: 10.3 months, 95% CI: 9.0-12.1; log-rank p = 0.0364). The stepwise model building process resulted in a final Cox model that included age, stage, BMI, practice region, and per label K dosing status. Patients dosed with K per label had a 27% lower risk for progressing to the next treatment or death, compared to those who had a lower K dose intensity (Adjusted HR: 0.73; 95% CI: 0.52-1.01; type 3 p = 0.058).
Conclusions
Our study found that less than a fifth of the patients received K dose per label, and a large majority received a lower K dose intensity. Patients dosed per label had a trend toward improved TTNT compared to patients treated with a lower K dose intensity. The reasons for treating patients with a lower K dose intensity are unclear and warrant further investigation.
Rifkin: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; McKesson Specialty Health: Employment. Amirian: McKesson Specialty Health: Employment. Medhekar: Amgen Inc.: Employment, Equity Ownership. Wilson: McKesson Specialty Health: Employment. Boyd: McKesson Specialty Health: Employment. Mezzi: Amgen, Inc.: Employment, Equity Ownership. Panjabi: Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal