Abstract
Background: Daratumumab has recently been approved in the United States (US) for patients with multiple myeloma (MM). In the CASTOR clinical trial of patients who had received at least one prior line of therapy, daratumumab plus bortezomib and dexamethasone (DVd) showed significant improvements in clinical efficacy compared to bortezomib plus dexamethasone (Vd) (Palumbo, NEJM, 2016). However, limited information is available on the cost-effectiveness of DVd. A decision-analytic model was developed to consider direct medical costs from a US payer perspective and to explore the cost-effectiveness of DVd versus Vd in previously treated MM and in a subgroup of patients with only one prior line of therapy (first relapse).
Methods: The model structure took the form of a partitioned survival analysis with three health states: progression-free survival (PFS), progressive disease, and death. A range of parametric survival functions were fitted to patient-level PFS data from the CASTOR trial with (1) selection based on the Akaike and Bayesian information criteria and (2) priority given to the external validity of extrapolations with conservative survival projections. Diagnostic plots were used to assess the validity of the proportional hazards assumption between DVd and Vd. Due to the number of data events and the follow-up time of the trial, overall survival (OS) was not estimated via parametric extrapolation of trial data but rather by its relationship to PFS, based on a retrospective analysis of approximately 23,000 MM patients (Felix, BMC Cancer, 2013). Drug costs were taken from wholesale acquisition costs (Red Book, 2017). Dosing information was extracted from the CASTOR trial. Drug-administration costs were sourced from Centers for Medicare and Medicaid Services, 2017 and health-state costs, subsequent therapy utilization patterns, and utility weights were sourced from published literature. Cost-effectiveness was estimated over a lifetime horizon, with costs and outcomes discounted 3% per annum. Incremental cost-effectiveness ratios were calculated, specifically the incremental cost per quality-adjusted life-year (QALY) gained and the incremental cost per life-year gained. One-way and probabilistic sensitivity analyses were conducted, as well as scenario analyses around parametric curve selection. All analyses were performed for the overall trial population and for the subgroup of patients with a first relapse.
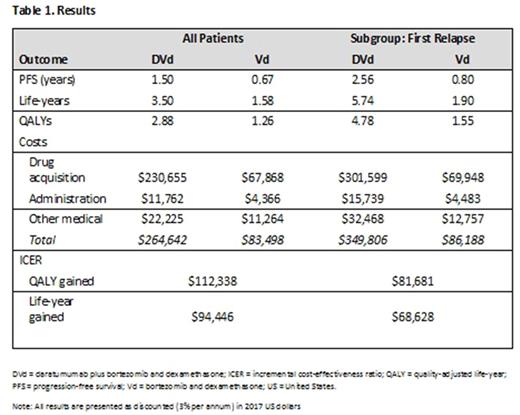
Results: The best-fit, conservative parametric function for all patients was the generalized gamma and for first relapse patients, Weibull. All results were presented as discounted values (Table 1). In the overall trial population, the mean PFS was estimated to be 1.50 and 0.67 years for DVd and Vd, respectively. DVd resulted in an average of 1.92 additional life-years (3.50 life-years - 1.58 life-years) and 1.62 more QALYs (2.88 QALYs - 1.26 QALYs) over Vd. Costs increased for DVd; the majority were related to drug acquisition of daratumumab and to longer time on treatment due to a longer PFS period. Incremental cost-effectiveness ratios in the overall population were $94,446 per life-year gained and $112,338 per QALY gained. Overall, survival (life-years), PFS, and QALYs were greater in the first relapse subgroup for both DVd and Vd than in the overall trial population. Mean PFS was estimated to be 2.56 and 0.80 years for DVd and Vd, respectively. DVd resulted in an average of 3.84 more life-years (5.74 life-years - 1.90 life-years) and 3.23 more QALYs (4.78 QALYs - 1.55 QALYs) over Vd. Incremental cost-effectiveness ratios in the first relapse subgroup were $68,628 per life-year gained and $81,681 per QALY. One-way and scenario analyses indicated the most sensitive parameters to be PFS distribution, the relationship between PFS and OS, discount rates, utility weights, and drug-acquisition costs. PSA was summarized in the form of scatter plots and cost-effectiveness acceptability curves.
Conclusion: Based on the range of plausible cost-effectiveness ratio thresholds ($183,000-$264,000 per life-year gained and $109,000-$297,000 per QALY gained) reported in the 2003 cost-effectiveness decision rules for the US (Braithwaite et al., Med Care, 2008), this analysis suggests that DVd is cost-effective compared to Vd in the treatment of previously treated MM. DVd offers a clinically superior and cost-responsible treatment option for previously treated MM.
Maiese: Johnson & Johnson, LLC: Equity Ownership; Janssen: Employment. Graham: RTI Health Solutions: Employment; Janssen: Research Funding. Hawe: RTI Health Solutions: Employment; Janssen: Research Funding. Le Moine: Janssen: Research Funding; RTI Health Solutions: Employment. Abraham: Janssen Oncology (Johnson & Johnson, LLC): Consultancy. Senbetta: Janssen: Employment; Johnson & Johnson, LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal