Abstract
Background: Unfractionated heparin (UFH) has been in clinical use for more than half a century. Due to the risk of hemorrhage in case of over dosage and the risk of thrombosis in case of under dosage, monitoring UFH treatments is mandatory. The activated partial thromboplastin time (aPTT) is the most widely used method as it is simple, cheap, and readily available. However, it is also poorly standardized and affected by many parameters, both analytic and pre-analytic, that are unrelated to the heparin effect. Particularly, the UFH-induced prolongation of aPTT is highly dependent on the reagent and analyzer used. In an effort to improve inter-laboratory agreement in the monitoring of UFH, the College of American Pathologists (CAP) recommends that the aPTT therapeutic range must be defined in each laboratory through correlation with a direct measure of heparin activity such as the factor Xa (FXa) inhibition assay. Such a strategy was endorsed years ago by the American College of Chest Physicians (ACCP) and the 9thACCP Consensus guideline recommends monitoring UFH treatments by using either the anti-FXa activity, with the therapeutic range between 0.30 and 0.70 IU/mL, or the anti-FXa-correlated aPTT. However, whether and to what extent this approach enhances the inter-laboratory agreement of UFH monitoring has been weakly evaluated, so far.
Objectives: To compare the inter-laboratory agreement of the anti-FXa activity and the anti-FXa-correlated aPTT for monitoring therapeutic UFH treatment, we conducted a cross-validation study among 3 accredited coagulation laboratories that used different technical conditions.
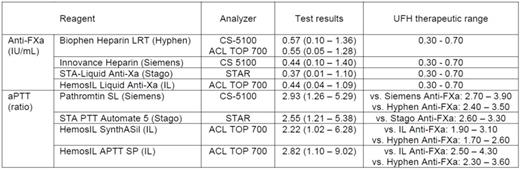
Patients and Methods: 125 inpatients treated with UFH were sampled in one of the centers. Blood was collected into evacuated polymer tubes containing 3.2% tri-Na citrate. Tubes were centrifuged twice within 2 h after collection, and plasma was stored frozen in aliquots at -70°C that were shipped in dry ice to the 2 other centers to be locally evaluated. Anti-FXa activity (in IU/mL) was evaluated using 4 chromogenic assays i.e. Biophen Heparin LRT (Hyphen BioMed), HemosIL Liquid Anti-Xa (Instrumentation Laboratory, IL), Innovance Heparin (Siemens), and STA-Liquid Anti-Xa (Stago). APTT (expressed as the patient-to-control ratio) was evaluated using 4 reagents i.e. HemosIL SynthASil and HemosIL APTT SP (IL), Pathromtin SL (Siemens), and STA-PTT Automate 5 (Stago). With some exceptions, participating centers used combinations of reagents and analyzers from the same manufacturer i.e. ACL TOP 700 (IL), CS-5100 (Siemens) and STAR (Stago).
Results: In the studied population, anti-FXa test results were found to be highly variable depending of the assay used, with the median activity ranging from 0.57 IU/mL with one reagent to 0.37 IU/mL with another, the two other reagents giving intermediate results (Table). Test results obtained using the different reagents were well correlated (r>0.91 in all cases) even though some comparisons performed according to Bland-Altman demonstrated unacceptable bias. If 0.30 to 0.70 IU/mL was used as the therapeutic range of anti-FXa activity, such a discrepancy in test results led to a lack of agreement as to whether a sample was subtherapeutic, therapeutic or supratherapeutic in more than 25% of the patients.
The median aPTT test result (ratio) ranged from 2.22 with the less sensitive to 2.93 with the most sensitive reagent. Using the anti-FXa-correlation method, aPTT therapeutic ranges were found to be highly different from one combination of reagent/analyzer to another (Table), and the same applied when correlations were made between one single aPTT reagent and different anti-FXa assays performed on the same analyzer. Consequently, agreement among all aPTT reagents was found in in less than 40% of the patient samples.
Conclusions: The reported discrepancy between test results evaluated using commercially available anti-FXa assays are consistent with the results of various national and international EQA programs, and clearly suggests a lack of standardization of that assay. As the consequence, the anti-FXa-correlated aPTT therapeutic range could be significantly impacted. In addition, the anti-FXa-correlation method did not appear to enhance interlaboratory agreement in UFH monitoring as it produces considerable disparity in UFH dosing decisions among different centers, although the clinical impact of this disparity is not known.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal