Abstract
Background
Spleen tyrosine kinase (SYK) is a nonreceptor protein kinase and a key mediator of immunoreceptor signaling implicated in the pathogenesis of B-cell malignancies. SYK has also been shown to directly bind and activate FMS-like tyrosine kinase 3 (FLT-3), a receptor tyrosine kinase that is commonly mutated in ~30% of pts with AML. TAK-659 is a potent and reversible dual inhibitor of SYK and FLT-3, which has demonstrated growth inhibition of cell lines and xenograft tumor models of AML origin, independent of FLT-3 mutation status. The primary objectives of this ph 1b/2 study in adult pts with R/R AML are to evaluate the safety, tolerability, and MTD/RP2D of TAK-659, as well as preliminary efficacy in both FLT-3-mutated and -wild type populations. Secondary objectives include evaluation of TAK-659 pharmacokinetics (PKs). This abstract presents updated data from ph 1b.
Methods
During 3+3 dose escalation, pts received oral TAK-659 60-160 mg daily (QD) or 80 mg twice daily (BID) in 28d cycles (C). Adverse events (AEs) were assessed per NCI-CTCAE v4.03. Response per IWG criteria for AML was assessed at the end of C1, C2, and C4. Blood samples for plasma PK assessments were collected pre-dose and at multiple times post-dose on d1 and d15 of C1. Two-compartment population PK modeling was applied to evaluate TAK-659 PK variability and predict steady-state trough concentrations after BID vs QD dosing. FLT-3 mutation status (wild type [WT], internal tandem duplication [ITD], or point mutation [D835Y]) was assessed via a PCR-based assay at a central laboratory. The effect of TAK-659 treatment on FLT-3-ITD phosphorylation was evaluated using a plasma inhibitory activity (PIA) assay (Levis et al. Blood 2006; 108:3477-83).
Results
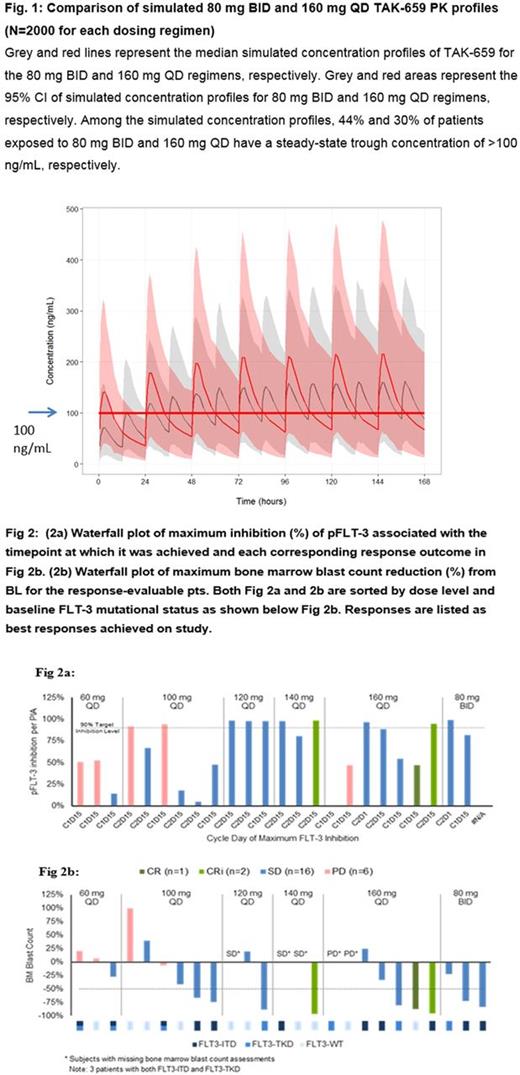
At data cut-off (May 24, 2017), 31 enrolled pts received TAK-659 QD 60 mg (n=4), 100 mg (n=7), 120 mg (n=4), 140 mg (n=5), 160 mg (n=9), and BID 80 mg (n=2). Median age was 67 yrs, 55% of pts were male, and 35% had ≥4 prior lines of therapy. 2 DLTs of Gr4 lipase elevation with corresponding Gr3 amylase elevation, and Gr3 bleeding were observed at 160 mg QD and 80 mg BID, respectively. Of all pts (n=31), 15 were FLT-3-WT, 8 had FLT-3-ITD, 5 had FLT-3-D835Y, and 3 had concurrent FLT-3-ITD/D835Y mutations based on central results. The most common any-grade drug-related AEs were asymptomatic lab changes including elevated AST (45%), ALT (29%), amylase (26%), and lipase (26%) levels. Gr≥3 drug-related AEs occurred in 13 (42%) pts including increased lipase (n=4), amylase (n=3), ALT (n=2) and AST (n=2) levels. 11 pts discontinued TAK-659 due to AEs and 15 pts died on study (1 drug related, multiple organ failure). Preliminary TAK-659 plasma PK (n=25, 60-160 mg) was characterized by rapid absorption (median tmax, 2 h), moderate variability in steady-state exposures (37.3% coefficient of variation for C1 d15 dose-normalized AUCtau), and mean accumulation of 1.91-fold after repeated QD dosing for 15 d. Simulated PK model results predicted 44% vs 30% of pts (N=2000) exposed to 80 mg BID vs 160 mg QD, respectively, would have a steady-state trough concentration of >100 ng/mL (Fig. 1), a level leading to >90% pFLT-3 inhibition per PIA; thus forming the basis for evaluating the BID dose schedule. 10 pts achieved >90% FLT-3 inhibition (Fig 2a). Early signs of clinical activity were observed in both FLT-3-mutated and WT pts (Fig 2b). 3 pts achieved response per IWG at higher dose levels: 1 CR (WT/160 mg) and 2 CRi (WT/140 mg; FLT-3 ITD/160 mg). Also, 4 other pts (2 FLT-3 ITD/100 mg; TKD/120 mg; TKD/160 mg) achieved >50% bone marrow (BM) blast reduction from baseline (BL) without BM recovery. 2 more pts (FLT-3 ITD/80mg BID) achieved >50% BM blast reduction from BL (92 to 15% and 81 to 22% respectively) after the data cut. The WT pt who achieved CR at C4 maintained CR through C10 (data cut-off) despite only achieving a maximal FLT-3 inhibition of 47% (C1d15), suggesting SYK inhibition may have contributed to this pt's response.
Conclusions
TAK-659 demonstrated an acceptable safety and PK profile as well as preliminary antitumor activity in R/R AML pts. TAK-659 BID may lead to higher trough exposure, offering longer duration of >90% pFLT-3 inhibition per PIA potentially required for FLT-3-driven efficacy. Early anti-leukemic activity has been observed in both FLT-3 mutant and WT pts at higher doses (140 and 160 mg QD) of TAK-659, supporting further study of SYK and FLT-3 as rational targets in AML.
Levis: Astellas Pharma Us: Consultancy, Research Funding; FujiFilm: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo, Inc.: Consultancy, Honoraria; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Morris: NIH: Patents & Royalties: NIH; BI, Merck: Membership on an entity's Board of Directors or advisory committees; BI, Merck: Speakers Bureau. Wise-Draper: Merck, BMS, AstraZeneca: Research Funding. Levy: Actinium Pharmaceuticals: Equity Ownership; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Takeda: Consultancy, Speakers Bureau. Burke: Novartis: Research Funding. Stumpo: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Kannan: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Sheldon-Waniga: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Wang: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Zhang: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Shou: Takeda Pharmaceuticals: Employment, Equity Ownership. Kaplan: Janssen, Seattle Genetics, Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal