Abstract
Introduction: Treatment of multiple myeloma (MM) has evolved tremendously, resulting in significant improvement of overall survival in patients. Frontline therapy can be broadly categorized into: proteasome inhibitor (PI)-based, immunomodulatory (IMiD)-based or PI+IMiD-based. Despite their widespread benefit, a considerable proportion of patients achieve suboptimal response to treatment. It would be clinically valuable to understand disease biology prior to initiating therapy and be able to predict the likelihood of response. Prior studies have attempted such a process in which response to PI and/or IMiD-based treatments were retrospectively examined in correlation to genomic testing from pre-treatment patient samples. The MMRF CoMMpass study provides a unique opportunity for interrogation of genes predictive of response to PI and/or IMiD-based regimens. Here, we performed a comparative analysis of genes previously reported to be associated with response to treatment or survival in MM patients with data recorded from the CoMMpass trial.
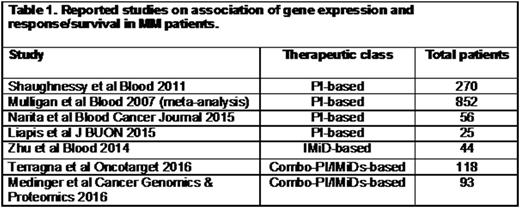
Methods: Previous studies with reported relationship of gene expression and response/survival outcome to PI and/or IMiD-based regimens were identified (Table 1). Next, we interrogated the CoMMpass database and abstracted patients by Best Response to First Line Therapy. Therapy class (PI and/or IMiD-based) was simplified to match the comparator studies in Table 1. We analyzed the genes reported in studies from Table 1 against our PI-based, IMiD-based and PI+IMiD-based CoMMpass patient cohorts. Logistics regression modeling and Bonferroni correction methods were applied adjusting for patient age, gender and ISS stage and gene odds ratios were calculated. An alpha of <0.00029 was considered significant due to multiple comparison of genes between the groups. We also compared mRNA expression for the genes identified in these 3 different treatment cohorts that had an alpha of ≤0.05 between patients classified as Responders (≥PR) and Non-responders (<PR).
Results: Of the 7 previous studies identified, 4 were PI-based, 1 was IMiD-based and 2 were PI+IMiD-based. From among these studies, a total of 173 genes (132 genes in the PI-based group; 25 in the IMiD-based group and 17 in the PI+IMiD-based group) were cross-analyzed in patients from the CoMMpass study. In the PI-based cohort, 10 genes with an alpha of ≤0.05 were associated with an increased likelihood of not responding to treatment, with OR ranging from 1.32-E5 (CNGA1) - 0.99 (CCND1). In the IMiDs-based treatment cohort, we identified 2 genes associated with an increased likelihood of response, with OR of 1.2 (RPL26L1 and SNRPD1) and an alpha of 0.02 and 0.03, respectively. In the PI+IMiD-based group, 22 genes (p≤0.05) with OR ranging from 2.28 (HIST1H3B) - 0.85 (WIPF2) were associated with the likelihood of response. Relative gene expression analysis in the different treatment CoMMpass cohorts showed that in PI-based treatment patients NMT1, PSMD4, MAP4K4, CNGA1, DR1 and UBFD1 were significantly decreased in responders (n=228) vs. non-responders (n=14) (p<0.05-<0.001). In the IMiD-based cohort, C14orf2 and RPL26L1 were significantly increased in responders (n=32) vs. non-responders (n=7, p<0.05). In the PI+IMiD treatment group, ACVC1C, CRYGS, FUNDC1, PSMB3, RPL26L1, HIST1H3B, PSMB10 and STUB1 were significantly increased whereas TMC8, MAPK4, CCNB1IP1, SAP18, ANK3, JAK1, RUNX3, IKZF1, CUL4B, HNRNPC and WIPF2 were significantly decreased in responders (n=361) vs. non-responders (n=18) (p<0.05-<0.001).
Conclusion: Our analysis investigates utility of the CoMMpass database to interrogate molecular data previously reported and shown to be associated with outcome to therapy in MM patients. While useful in comparison of relative gene expression changes and mutation data associated with patients receiving PI, IMiD or PI+IMiD-based therapy, cross-comparison of data from prior studies should be performed with caution considering variability in methodology and patients who have received treatment at different stages of their disease vs. frontline only in the CoMMpass dataset. Overall our analysis highlights the potential of CoMMpass data to develop future prospective studies with biomarkers and/or predictive models for MM therapeutics.
Acknowledgments: We would like to thank Dr. Daniel Auclaire and the MMRF CoMMpass Network.
Sher: LAM Therapeutics, Inc: Research Funding. Ailawadhi: Pharmacyclics: Research Funding; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal