Abstract
Introduction: in clinical trials, surrogate endpoints allow for an earlier measurement of a treatment effect and with smaller sample size compared with conventional endpoints. After allogeneic hematopoietic stem cell transplantation (HSCT), endpoints like progression-free survival (PFS) or the recent GvHD-free/relapse-free survival (GRFS) do not account for the late mortality that is observed in patients with chronic GvHD requiring long periods of immune suppression therapy (IST), this latter responsible for severe infection and secondary malignancies.
Methods: we test here a new estimate, the "current IST-free/relapse-free survival" in 326 patients affected by acute leukemia who received HSCT at Lyon transplant center from Jan 2006 to Dec 2014. The probability of being IST- and relapse-free at 6 and 12 months was calculated on the series and Kendall's tau correlation was performed with OS, in order to evaluate the correlation between this new endpoint and survival. Patients were included according to the following criteria: age > 18y, diagnosis of acute leukemia, first allogeneic HSCT. All the main variables were extracted from the EBMT database ProMISe, with the addition of the date(s) of beginning and end of IST (retrieved from patients' charts), whenever it was administered for GvHD prophylaxis or treatment. The same variables were tested for any association with OS and /or current IST-free/relapse-free survival.
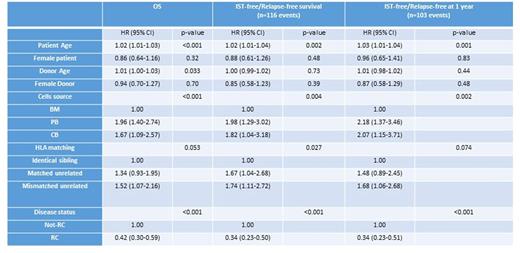
Results: the 1- and 5-year OS for the entire series was 66.1% (95% CI: 60.6-70.9) and 50.4% (95% CI: 44.6-55.8), respectively. The probability of being alive, IST- and relapse-free was 70.4% (95% CI: 64.9-75.2), 53.6% (95% CI: 44-62.3) and 32.7% (95% CI: 21.6-44.3) at 6 months, 1 and 2 years, respectively. Kendall's tau correlation analysis was as follows: between IST-free/relapse-free at 6 months and OS at 5 years: Rho=0.41 (95% CI:0.35-0.47); p<.001; between IST-free/relapse-free at 1 year and OS at 5 years: Rho=0.42 (95% CI: 0.36-0.48); p<.001. Of note, among the 103 patients who were not IST-free/relapse-free at 1 year, 100 (97.1%) died within 5 years while among patients IST-free/relapse-free at 1 year only 30.7% died within 5 years. Variables significantly associated with the new endpoint and OS are shown in the Table 1.
Conclusions: the "current IST-free/relapse-free survival", showed to be predictive of 5-year OS, with 97% of patients not IST- nor relapse-free at 1 year who subsequently died and 69.3% of those IST- and relapse-free who were still alive at 5 years. After further explorations, this new estimate is expected to overcome the current limitation of PFS and GRFS in predicting late mortality due to GvHD requiring IST for >1 year after HSCT and should be validated in a larger multicenter study.
Table 1. Variables tested for the association with the new endpoint and with OS
Crocchiolo: MolMed S.p.A.: Employment. Nicolini: Incyte Biosciences: Honoraria, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal