Abstract
Background: Rituximab plus CHOP (R-CHOP) remains the standard of care treatment in patients with previously untreated diffuse large B cell lymphoma (DLBCL) despite a risk of about 25-30% of treatment failure during the first 2 years (Cunningham et al Lancet, 2013,). Obinutuzumab (GA101) is a glycoengineered, type II anti-CD20 monoclonal antibody designed to optimize direct cell death induction and antibody-dependent cellular cytotoxicity. GAINED (NCT01659099) is a phase III randomized prospective trial that aimed to randomly compare obinutuzumab to rituximab combined with 4 cycles of intensified chemotherapy as induction treatment (ACVBP14 or CHOP14, according to local center practice) using a PET driven consolidation strategy.
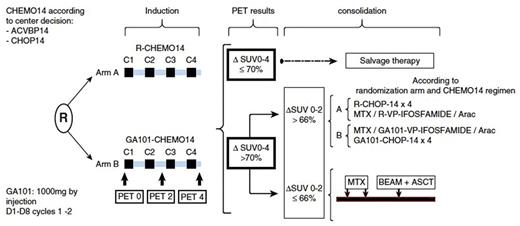
Methods: Eligible patients were aged 18-60 y with an aa-IPI 1-3 previously untreated CD20+ DLBCL. Patients were randomized between obinutuzumab (1000mg iv on D1 and D8 cycle 1 and 2, and D1 cycle 3-4) or rituximab (375mg/m2 iv D1) in combination with 4 cycles of ACVBP14 or CHOP14. Randomization was stratified according to aaIPI (1 vs 2-3) and chemotherapy regimen. PET was performed at baseline, after 2 (PET2) and 4 (PET4) cycles of induction immunochemotherapy and centrally reviewed using ΔSUVmax method (Casasnovas et al, 2011 Blood) (See Fig 1). PET2-/PET4- patients were assigned to immunochemotherapy consolidation with either rituximab or obinutuzumab according to the randomization arm; PET2+/PET4- patients received autologous stem cell transplant and PET4+ patients were treated according to investigator' choice. The primary endpoint was 2y-EFS defined by PET positivity after 2 or 4 induction cycles, progression or relapse, modification of planned treatment not due to progression (including radiotherapy) or death of any cause. Safety, response rates, progression free survival (PFS) and overall survival were secondary objectives. Herein, we present the result of the third planned interim analysis.
Results: From Sept 2012 to Jul 2015, 670 patients were enrolled. Median age was 48y and 55.7% of patients were male. 41.8% of patients presented with IPI >2 at diagnosis. In all, 336 were randomized in the obinutuzumab plus chemotherapy arm (G-ACVBP, n= 165; G-CHOP, n=171) and 334 in the rituximab plus chemotherapy arm (R-ACVBP, n= 162; R-CHOP, n= 172). Patients' characteristics (age, PS, Ann Arbor stage, LDH level, IPI score) were similar between the two arms except a higher proportion of male patients in the obinutuzumab arm (60.4% vs 50.9%; p<0.016). Regarding toxicity, 455 SAEs have been reported including 250 (in 140 pts) in the obinutuzumab arm and 205 (in 107 pts) in the rituximab arm. Fatal AEs were reported in 10 patients in the obinutuzumab arm (in the G-ACVBP arm in 7 cases) and 1 in the rituximab arm. At the cut-off date (August 1st, 2016), the median follow-up was 25.2m (range: 24.28-26.2) and 284 patients (42.4%) had an event. The results of the planned third interim analysis were presented to the DSMC members who recommended stopping the trial. The stratified 2y-EFS were not statistically different between the two arms (p= 0.1321, above the futility bound of 0.069). The 2y-PFS for aa-IPI 1 patients were 61.3% (95%CI; 48.9-71.6) for G-CHOP versus 59.9% (95%CI; 47.6-70.2) for R-CHOP, 62.7% (95%CI; 50.6-72.5) for G-ACVBP versus 61.9% (95%CI; 49.8-71.9) for R-ACVBP. The 2y-PFS for aa-IPI 2-3 patients were 56.1% (95%CI; 45.4-65.4) for G-CHOP versus 51.7% (95%CI; 41.3-61.1) for R-CHOP, 61% (95%CI; 49.7-70.4) for G-ACVBP versus 56.8% (95%CI; 45.8-66.4) for R-ACVBP. The unstratified 2y-EFS were 60% (95%CI; 54.4-65.1) for patients in the obinutuzumab arm vs 57% (95%CI; 51.5-62.2) in the rituximab arm (p = 0.1296; HR=0.876; 95%CI, 0.694 to 1.105).
Conclusions: Obinutuzumab plus chemotherapy is not superior to rituximab plus chemotherapy delivered every 14 days in young DLBCL patients. Cell of origin data are currently investigated using nanostring technology and will be presented at the time of the meeting.
Casasnovas: Janssen: Consultancy, Honoraria; MSD: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding. Salles: Janssen: Consultancy, Honoraria; morphosys: Consultancy, Honoraria; BMS: Consultancy; Servier: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding. Morschhauser: Roche: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Tilly: Immunogen: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ribrag: BMS: Consultancy; Novartis: Consultancy. Lamy: Roche: Consultancy, Honoraria. Thieblemont: Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Haioun: Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sandoz: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; GILEAD: Consultancy, Honoraria; PFIZER: Consultancy, Honoraria. Fornecker: Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Feugier: Roche: Consultancy, Honoraria, Research Funding. Delarue: Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Bonnet: Takeda: Other: advisory board. Cartron: Sanofi, BMS, Jansen, celgene, Roche, Gilead: Equity Ownership; Celgene: Consultancy, Employment; Roche: Consultancy, Equity Ownership, Honoraria, Research Funding. Molina: Merck Serono: Honoraria; Novartis: Honoraria; Takeda: Other: travel support to the ASH meeting. Le Gouill: Roche: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal