Abstract
Bone-marrow transplantation (BMT) offers curative potential for patients with high-risk hematologic malignancies, but the post-transplantation period is characterized by profound immunodeficiency. Delayed immune reconstitution is an important contributor to transplant-related morbidity and mortality (Maury et al., Br J Haematol, 2001; Small et al., Blood, 1999). Thus, understanding the mechanisms by which the immune system reconstitutes and how environmental exposures might influence this process is an important objective in improving outcomes after BMT.
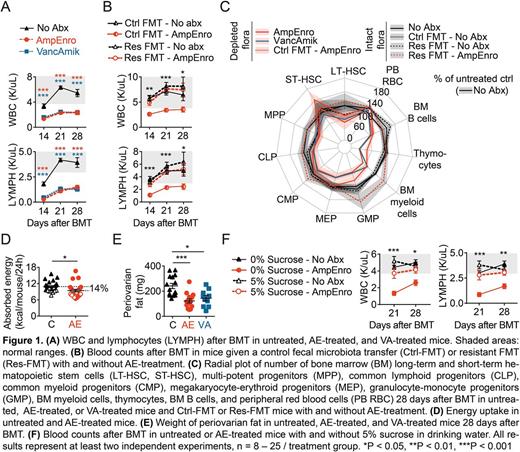
The intestinal microbiota shapes both mucosal and systemic immunity and contributes to steady-state hematopoiesis (Belkaid and Hand, Cell, 2014). To determine the role of the intestinal flora in hematopoietic reconstitution following BMT, we performed syngeneic BMT after lethal irradiation in mice with an intact intestinal flora (control, C/No abx) and in mice treated with two different antibiotic (abx) regimens administered in the drinking water: ampicillin + enrofloxacin (AmpEnro/AE) or vancomycin + amikacin (VancAmik/VA). While both regimens depleted the intestinal flora 1000-fold, ampicillin and enrofloxacin are both relatively well absorbed in the intestine while vancomycin and amikacin both have poor oral bioavailability with negligible systemic effects. BMT recipients treated with either of the abx regimens had lower white blood cell (WBC) counts and, in particular, lower lymphocyte counts compared to untreated BMT recipients (Fig 1A). Fecal microbiota transfer (FMT) with an abx-resistant intestinal flora (Caballero et al., Cell Host Microbe, 2017) rescued the impaired hematopoietic reconstitution in AE-treated mice (Fig 1B). These data rule out a direct inhibitory effect of abx on hematopoietic recovery. When we examined multiple compartments of the hematopoietic cascade we observed that flora depletion significantly impaired hematopoiesis specifically in the lymphoid and myeloid compartments of the bone marrow, thymus, and peripheral blood, while the hematopoietic stem and progenitor compartment and erythroid lineage were largely unaffected. These data are summarized in a radial plot (Fig 1C) in which each compartment of the hematopoietic cascade is a spoke and the distance from the center is proportional the cell count.
Treatment of mice with AmpEnro or VancAmik also reduced dietary energy uptake (Fig 1D), resulting in reduction of visceral adipose tissue (Fig 1E). The mice are normally fed a standard chow diet based on complex plant carbohydrates. To test if reduced uptake of calories in flora-depleted animals contributed to impaired hematopoiesis post-BMT, we supplemented the drinking water with 5% sucrose, a carbohydrate source that can be directly utilized by the host in the absence of microbiota metabolism. Sucrose supplementation increased peripheral WBC counts and lymphocyte counts in AE-treated mice (Fig 1F), indicating that a critical function of the intestinal microbiota in supporting hematopoiesis post-BMT is to improve dietary energy uptake.
We conclude that apart from specific and direct effects of the microbiota on immuno-hematopoiesis as previously described (Balmer et al., J Immunol, 2014; Clarke et al., Nat Med, 2010; Mazmanian et al., Cell, 2005), the intestinal flora can contribute to post-transplant hematopoietic reconstitution through improved dietary energy uptake.
Peled: Seres: Research Funding. Jenq: Seres: Research Funding. van den Brink: Therakos Institute: Other: Speaking engagement; Jazz Pharmaceuticals: Consultancy; PureTech Health: Consultancy; Seres: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal