Abstract
Background
Maintenance is a part of standard practice in multiple myeloma patients receiving induction followed by autologous stem cell transplantation (ASCT). Various regimens including thalidomide, lenalidomide and proteasome inhibitor are used as maintenance treatment. However, few prospective trials have been performed evaluating maintenance treatment in Asian myeloma patients. In this prospective multi-center trial, we evaluated the efficacy and toxicity of thalidomide-based maintenance therapy in Korean myeloma patients.
Methods
We conducted an open-label, prospective, multicenter phase II study. Patients with newly diagnosed multiple myeloma who underwent induction followed by ASCT were enrolled. Patients should not have refractoriness to previous thalidomide if the drug is included in induction regimen, and should have achieved at least partial response (PR) after ASCT. Patients received maintenance treatment of 100mg thalidomide daily for 28 days and 40mg dexamethasone for 4 days. The maintenance therapy was continued for one year unless there were disease progression, unacceptable toxicity, or consent withdrawal. The primary endpoint was 1-year event-free-survival (EFS). Progression free survival (PFS), overall survival (OS), drug tolerability and safety were also evaluated.
Results
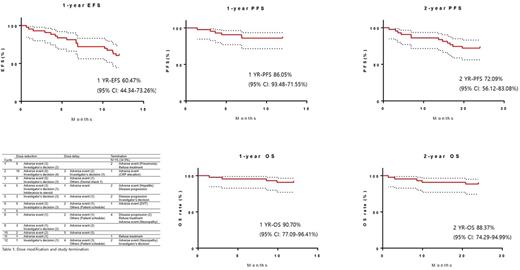
A total of 43 patients were enrolled. One-year EFS rate was 60.47% (95% confidence interval (CI), 44.34-73.26%). Progression-free survival (PFS) was 86.05% at 1-year and 72.09% at 2-year. Overall survival (OS) at 1-year and 2-year was 90.70% and 88.37%, respectively. Among 21 patients who did not acquire complete remission (CR) after ASCT, 4 patients reached CR after maintenance therapy. The maintenance treatment was discontinued in 15 patients (34.9%). The most common cause was adverse events (6 patients) followed by disease progression (4 patients). Rest of the patients terminated the treatment by patient's withdrawal (3 patients) and investigator's decision (2 patients). In each cycle, up to 23.3% of patients experienced dose reduction or delayed medication. Two leading causes of the schedule change were the adverse event and physician's decision. Grade 3 or 4 hematologic adverse events (AE) was upper respiratory infection (20 patients, 46.5%) followed by peripheral neuropathy (19 patients, 44.2%). Among the patients with neuropathy, 2 patients showed more than grade 3 symptom. Two patients showed serious adverse events (pneumonia and fulminant hepatitis). Thromboprophylaxis was performed in 37 patients using aspirin and one patient developed deep vein thrombosis during maintenance.
Conclusions
Thalidomide and dexamethasone maintenance for transplant eligible, newly diagnosed Korean multiple myeloma patients showed 1-year EFS and OS rate of 60.47% and 90.70% which is similar to the reports from western countries. Thalidomide and dexamethasone were tolerable as maintenance in Asian patients, although high hematologic toxicity draws concern. We concluded that thalidomide-based maintenance therapy is one of suitable option in Asian transplant eligible, newly diagnosed multiple myeloma patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal