Abstract
Background: CLL is a heterogeneous disease with several prognostic models proposed for risk stratification. The validated CLL-IPI model (Lancet Oncology; 2016) integrates weighted key markers: TP53 dysfunction (del17p or TP53 mutation) = 4 points, unmutated IGHV= 2 points, β2M >3.5 mg/L = 2 points, Binet stage ≥B or Rai stage ≥I = 1 point, and age >65 years = 1 point. The CLL-IPI divides patients into 4 risk groups with differential survival outcomes, but it was developed based on patients receiving chemoimmunotherapy, not novel agents, such as ibrutinib. Whether the CLL-IPI applies to patients receiving ibrutinib is unknown. We sought to examine how the CLL-IPI impacts pts receiving ibrutinib monotherapy in the front line setting.
Patients and Methods: A retrospective cohort analysis of CLL pts treated with front-line ibrutinib across 19 community and academic centers was conducted. We analyzed demographics, prognostic factors, CLL-IPI scores, and survival outcomes. The primary endpoint was progression-free survival (PFS) measured from the time of ibrutinib initiation until CLL progression, death, or last follow up as estimated by the Kaplan Meier method. Using Cox regression, we examined the CLL-IPI score and its individual components as predictors of PFS. Descriptive statistics were used for demographic data.
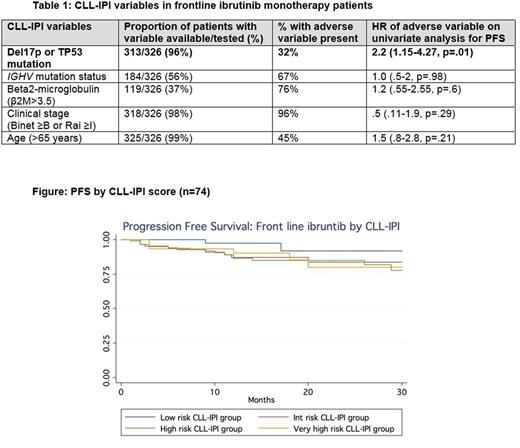
Results: A total of 326 CLL pts treated with front-line ibrutinib monotherapy were included in this analysis. Median age at ibrutinib initiation was 68 years (range, 36-96), 63% were males. Median follow up on ibrutinib was 12 months (range, 1-76). Frequency of the CLL-IPI adverse variable testing and presence is displayed in Table 1. Of pts tested, 32% had TP53 dysfunction (del17p or TP53 mutation), 67% were IGHV unmutated, 76% had β2M >3.5 mg/L, 96% had advanced clinical stage, and 45% were age >65 years. In total, 79 pts (24%) had all variables available to accurately calculate the CLL-IPI. Of these, 2.5% were low risk, 20% intermediate risk, 49.5% high risk, and 28% very high risk. The 12 month PFS on frontline ibrutinib for intermediate, high, and very high risk groups was 86%, 94%, and 94%, respectively. These differences were not significant (Figure). On univariate analyses of individual CLL-IPI variables, only the presence of del17p and/or TP53 mutation was significantly associated with inferior PFS (HR 2.2, p=.01).
Conclusions: In the largest cohort of 326 frontline ibrutinib-treated CLL pts reported to date, only 79 patients had all CLL-IPI variables available. β2M was missing in 63% and IGHV mutational analysis was missing in 44%. Contrary to pts treated with chemoimmunotherapy, CLL-IPI did not distinguish 12 month PFS in ibrutinib-treated pts. Only del17p and/or TP53 mutation was associated with inferior PFS. Our analysis suggests the need for different prognostic models in pts treated with ibrutinib and confirms that testing of all CLL-IPI prognostic variables is not commonly performed in the real world.
Brander: TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy. Pagel: Gilead: Consultancy; Pharmacyclics: Consultancy. Tam: Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding. Lamanna: Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lansigan: Seattle Genetics: Consultancy; Spectrum Pharmaceuticals: Consultancy, Research Funding. Shadman: Gilead: Research Funding; AbbVie: Other: advisory board; Emergent: Research Funding; PLEXXIKON: Research Funding; Acerta Pharma: Research Funding; Celgene: Research Funding; TG Therapeutics: Research Funding; Merck: Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Other: advisory board, Research Funding. Ujjani: Genentech: Consultancy; Abbvie: Research Funding, Speakers Bureau; Gilead: Consultancy; Pharmacyclics: Consultancy, Research Funding. Skarbnik: Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Novartis: Speakers Bureau. Cheson: AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy; Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support . Barr: AbbVie: Consultancy, Research Funding; Celgene: Consultancy; Infinity: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Seattle Genetics: Consultancy; Gilead: Consultancy. Dwivedy Nasta: Takeda: Research Funding; Incyte: Research Funding; Immunogen: Research Funding. Furman: Janssen: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Gilead: Consultancy; Abbvie: Consultancy, Honoraria; Genentech: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy. Mato: Janssen: Consultancy; Kite: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; DTRM: Research Funding; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy; Acerta: Research Funding; Regeneron: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal