Abstract
Epstein-Barr virus (EBV) is associated with many types of lymphoproliferative disorders. EBV-coded latent membrane protein 2A (LMP2A) functions in promoting B cell survival partly through Bruton's tyrosine kinase (Btk) -dependent signaling pathway. In the present study, we investigated whether a Btk inhibitor, Ibrutinib, shows therapeutic efficacy for EBV-related lymphoma. Ibrutinib has been already approved as a therapeutic drug for several B cell malignancies, such as chronic lymphocytic leukemia. Hence, once it shows efficacy, Ibrutinib could be quickly moved to clinical usage.
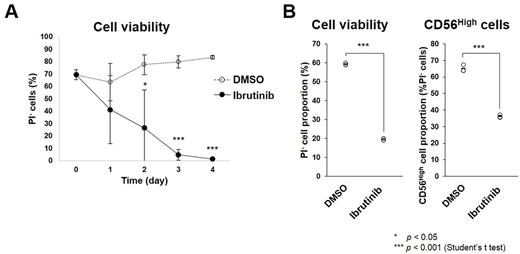
First, we examined the effects of Ibrutinib on B cells transformed by EBV infection in vitro, called lymphoblastic cell lines (LCLs). We cultured LCLs in the presence or absence of Ibrutinib and analyzed cell survival using flow cytometry. As a result, Ibrutinib treatment decreased survival rate of the LCLs compared with a vehicle (Fig. A). Indeed, Btk phosphorylation was detected in the LCLs by western blot analysis. Collectively, Btk signal inhibition by Ibrutinib is probably lethal for EBV-LCLs. LCLs are classified as latency type III and have a polyclonal nature like lymphoproliferative disorders in post-transplant and HIV-associated immune suppressed individuals. Hence, Ibrutinib possibly be applicable to treatment for such disorders. To examine whether Ibrutinib is effective in vivo, we are now planning to administer Ibrutinib to EBV-infected humanized mice, which can be used as a model for EBV-related lymphoproliferative disorders (Yajima et al., J. Infect. Dis. 2008).
Further, we obtained peripheral blood mononuclear cells (PBMCs) from EBV+ aggressive NK cell leukemia (ANKL) patient. Approximately 90% of the PBMCs were positive for NK cell marker CD56 and hence probably be leukemia cells. First, we examined in vitro effects of Ibrutinib on the PBMCs. The PBMCs were cultured in the presence or absence of Ibrutinib, and survival rate was analyzed using flow cytometry. Results showed that Ibrutinib treatment decreased survival of CD56High cells (Fig. B). Next, we examined Btk phosphorylation in the PBMCs using western blot. Unexpectedly, even total Btk was not detected. This suggests Ibrutinib-induced lethality of the ANKL cells is dependent on other pathways. Ibrutinib could target other kinases than Btk including those reported to be expressed in NK cells, such as ITK, TXK, and JAK3. Hence, it is possible that Ibrutinib decreases the ANKL cells through inhibiting these kinases.
Next, we tried to establish a mouse model of ANKL xenograft for in vivo assays. We transplanted the PBMCs from ANKL patient into immunodeficient NOG mice after X-ray irradiation. Around 2 weeks after the transplantation, a large number of the transplanted PBMCs were detected at least in spleen and bone marrow. These ANKL cells in spleen and bone marrow could be successively transplanted into other NOG mice. After transplantation, the recipient mice gradually decreased their body weight and died in several weeks. Now, we are analyzing these mice in detail and are investigating the effects of Ibrutinib administration on the survival of these mice.
Collectively, we found that Ibrutinib inhibits survival of EBV+ LCLs and ANKL cells in vitro . This raises a possibility that Ibrutinib can be used for EBV-related lymphoproliferative disorders and EBV+ ANKL, although we should confirm the effectiveness of Ibrutinib in vivo .
Kotani: Janssen Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal