Abstract
Background: Children with ITP for ≥6 months who completed a romiplostim phase 1/2 or phase 3 study could enroll in an open-label extension study; final data are presented here.
Methods: Patients enrolled at 28 sites in the US, Canada, Spain, and Australia. All patients received weekly SC romiplostim. The initial dose was the parent study final dose or 1 µg/kg for those previously receiving placebo, adjusted weekly by 1 µg/kg/week from 1-10 µg/kg to target platelet counts of 50-200×109/L. Incidence of adverse events (AEs) was the primary endpoint.
Results: Sixty-six patients entered the extension; 1 withdrew consent before treatment and 65 received romiplostim for ≤7 years. At baseline, median (min-max) age was 11 (3-18) years; 56% were female; 61% were white, 14% African American, 14% Hispanic/Latino, 9% Asian, and 3% other; 9.1% had prior splenectomy. Median (min-max) baseline platelet count was 27.5 (2-458)×109/L.
Median (min-max) treatment was 135 (5-363) weeks for a total of 182 patient-years, or 2.8 years per patient. Median (min-max) average weekly dose was 4.8 (0.1-10.0) µg/kg, including escalation to a stable dose; 20 patients started on 1 μg/kg, including 15 (23%) of whom had been on placebo in the previous study. All 65 patients received their doses per protocol >90% of the time; 21 patients missed ≥1 dose for noncompliance a total of 65 times. Reasons for discontinuing romiplostim (n = 28, 42%) included consent withdrawn (n = 10), required other therapy (n = 6), noncompliance (n = 4), per protocol (n = 3), administrative decision (n = 2), treatment not needed (n = 1), and AE (n = 2) (asthenia, headache, dehydration, and vomiting in one patient and anxiety in the other; per investigators, none were treatment related). Thirty-seven (56%) patients received romiplostim until study end. Fifty-four serious AEs occurred in 19 patients but were treatment related in only 1 patient (concurrent grade 4 thrombocytopenia, grade 3 epistaxis, and grade 2 anemia). Bleeding AEs occurred in 57 patients; 3 AEs were deemed treatment related (injection site hemorrhage, injection site bruising, and epistaxis). The most frequent bleeding AEs were contusion (51%), epistaxis (49%), petechiae (31%), and gingival bleeding (20%). No thrombotic events were reported. Bone marrow biopsies were performed for 2 patients with additional cytopenias; both had iron-deficiency anemia. Upon leaving the study to receive other therapy, one patient had anti-romiplostim neutralizing antibody (Ab) which was absent on retesting 3 and 6 months later. No patients had anti-TPO neutralizing Ab.
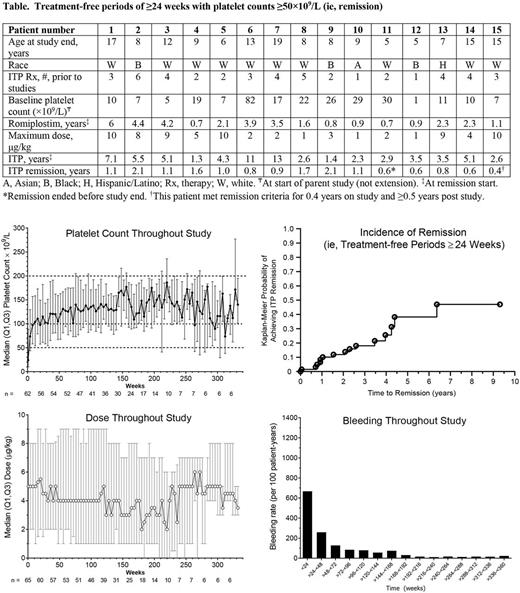
From week 2 on, median platelet counts remained >50×109/L; median platelet counts were >100×109/L from week 24 to week 260 (Figure). Nearly all (94%, 61/65) patients had ≥1 platelet response (platelet counts ≥50×109/L, excluding counts ≤4 weeks after rescue medication). The median (Q1, Q3) % months responding was 93% (68%, 100%) and the median (Q1, Q3) number of months responding was 30 (13, 43). Most (72%, 47/65) patients had a platelet response ≥75% of the time and over half (58%, 38/65) had a platelet response ≥90% of the time. Sixty (92%) patients (or caregivers) self-administered romiplostim. Twenty-three (35%) patients received rescue medications; usage was highest in the first few months. Fifteen (23%) patients had treatment-free periods of platelet counts ≥50×109/L for ≥24 weeks (here also called remission; Table, Figure). At the start of the treatment-free periods, these patients (9 girls, 6 boys) had had ITP for a median (min-max) of 3.5 (1.3-13) years (none with prior splenectomy) and had received romiplostim for 2.1 (0.7-6) years. All 15 of these patients had platelet counts over 100×109/L for ≥3 months and 12/15 for ≥6 months. The median (min-max) duration of being ≥100×109/L for these 15 patients was 42 (13-109) weeks. Of baseline characteristics such as sex, platelet counts, ITP duration, and number of past ITP treatments (1, 2, 3, >3), only age < 6 years was predictive of developing treatment-free periods ≥24 weeks (p=0.0035).
Conclusion: Seven years of data from this open-label extension study of romiplostim in children with ITP show that >90% of children achieved a platelet response with romiplostim, most responding ≥75% of the time. Romiplostim was mostly well tolerated. Surprisingly, 23% of these patients with longstanding ITP (median 3.5 years) were able to discontinue all ITP medications (including romiplostim) for at least 6 months.
Tarantino: Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxalta: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Reviews grants for Pfizer; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Bussel: Boehringer Ingleheim: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Honoraria, Patents & Royalties; Momenta: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau. Despotovic: Schell Cooley LLP: Other: Expert witness; Sanofi: Consultancy. Carpenter: Amgen Inc.: Employment, Equity Ownership. Mehta: Amgen Inc.: Employment, Equity Ownership. Eisen: Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal