Abstract

Background: The treatment of pediatric acute myeloid leukemia (AML) generally consists of near-myeloablative therapy to induce remission. However, the benefit of higher doses of induction therapy is not clearly established and has been recently challenged. The clinical impact of reducing the intensity of induction therapy for children with AML remains unknown.
Methods: 140 children younger than 15 years of age with de novo AML were enrolled in this study. Forty-six patients received low-dose chemotherapy (LDC) concurrently with granulocyte colony-stimulating factor (G-CSF) for the first induction course while 94 patients received standard-dose chemotherapy (SDC). All patients received similar standard postremission treatment. The median age of the 82 males and 58 females was 81 months (5-170 months). Clinical outcomes of children were compared between the two groups, and molecular response was assessed by whole-exome and targeted sequencing.
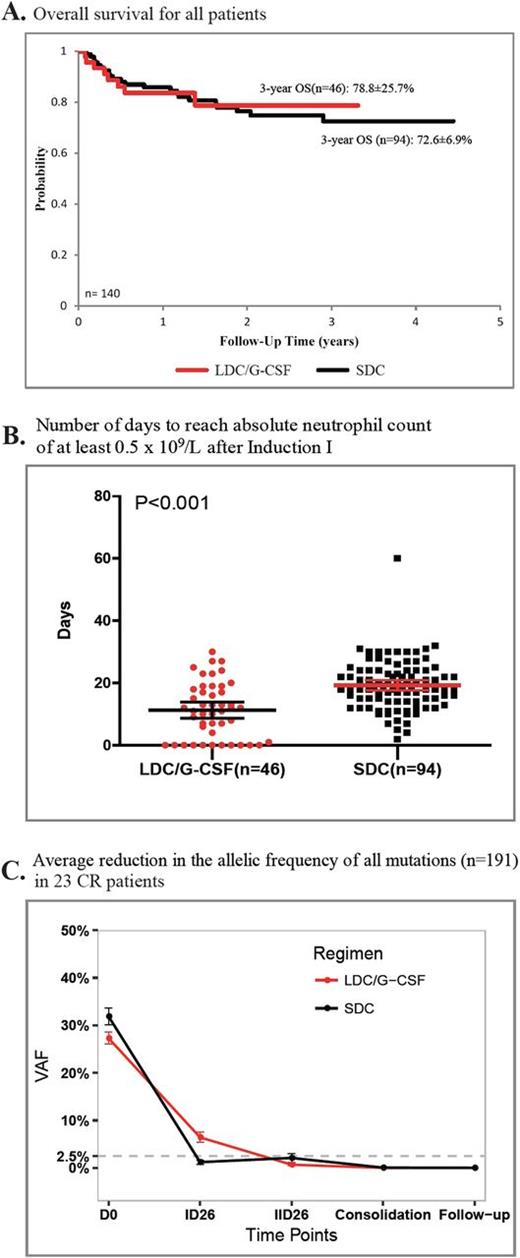
Results: Complete remission (CR) after 2 courses of induction was achieved in 87.0% (40 of 46) and 85.1% (80 of 94) of patients receiving LDC/G-CSF and SDC at first induction, respectively (P=0.80). There were no significant differences in the 3-year event-free survival (65.5% vs. 66.9%; P=0.99) and overall survival (78.8% vs. 72.6%, P=0.96) of patients receiving LDC/G-CSF or SDC at first induction, respectively. Risk of relapse and treatment with hematopoietic stem cell transplantation, but not type of regimen used in induction, were significantly associated with outcome. Recovery of white blood cell (WBC) and platelet counts was significantly faster in patients receiving LDC/G-CSF than those receiving SDC [11.5 vs. 18.5 days for WBC count (P <0.001); 15.5.0 vs. 22.0 days for platelet count (P <0.001)].
Of 191 leukemia cell mutations identified by exome sequencing at diagnosis in 23 patients achieving CR, 95.8% (93 of 97) and 91.7% (55 of 60) were cleared [variant allele frequency (VAF)<2.5%] after 2 courses of LDC/G-CSF or SDC, respectively (P=0.45). All 51 of the reported leukemia-associated recurrently mutated genes were cleared after 2 induction courses, regardless of the regimen received. Notably, of all mutations detected at diagnosis, all but 1 mutation were undetectable (VAF<1%) in follow-up samples (0-19 months off therapy). The persistent mutation (CACNA1G; VAF, 1.1%) was detected in the follow-up sample of a child receiving the SDC regimen at the 11-month off-therapy visit, who relapsed 4 months later.
Conclusions: Children with AML receiving LDC/G-CSF for remission induction had similar outcomes but experienced significantly less toxicity than those receiving SDC. Genome-wide mutational clearance in CR patients revealed that both regimens resulted in similar levels of molecular remission. Thus, we recommend LDC/G-CSF as an alternative approach to remission induction in pediatric AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal