Abstract
Novel therapeutic approaches are urgently needed for Acute Myeloid Leukemia (AML). Currently only 20% of patients over age 56 survive 2 years leading to an enormous unmet need for novel approaches. Unfortunately, there has not been a change in standard AML agents in over 40 years. We and others previously identified that targeting GSK3 in AML is a promising therapeutic strategy as it has high activity on leukemic cells, but does not impair the growth of normal hematopoietic progenitor cells. One major reason for the selective responsiveness of AML and normal hematopoietic cells may be due to the fact that GSK3β protein is markedly different between these two conditions. In AML, GSK3β is highly overexpressed, activated, and can be found at high levels in the nucleus. Our cell, animal and patient biomarker studies suggest that this nuclear pool of GSK3β that is not found in normal hematopoietic cells can drive AML progression and drug resistance.
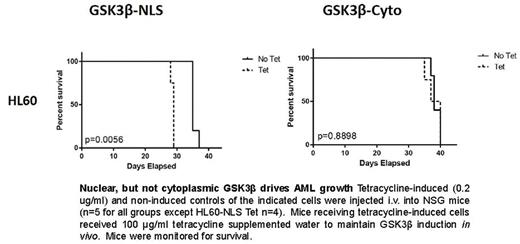
GSK3β protein is markedly elevated universally and aberrantly localized to the nucleus in all AML samples tested (>100 samples) by flow cytometry as well as western blot. Nuclear GSK3β has unique access to specific target proteins such as nuclear transcription factors leading to different effects of nuclear and cytoplasmic GSK3β on cellular processes. In order to simultaneously quantify the nuclear localization and expression of GSK3β in AML (n=86) as compared to normal hematopoietic cells (n=10), imaging cytometry was utilized. This cytometric analysis along with traditional fluorescent microscopy identified a large pool of nuclear GSK3β that does not exist in normal hematopoietic cells. To characterize the function of nuclear GSK3β, we developed AML cell lines that expressed inducible and targeted (nuclear or cytoplasmic) forms of GSK3b. Interestingly, the expression of nuclear, but not cytoplasmic targeted GSK3β increased colony formation of AML cells (ex. 38% more colonies formed in HL-60 cells with nuclear as opposed to cytoplasmic GSK3β induction, p<0.001). To further analyze the impact of GSK3β localization in AML, we investigated the ability of nuclear and cytoplasmic GSK3β to both promote AML disease progression and chemotherapy resistance in vivo in immunodeficient mice . Nuclear, but not cytoplasmic GSK3β induction in vivo led to enhanced AML growth and doxorubicin resistance using two AML cell lines (ex. See figure). Finally, nuclear GSK3β localization has clinical significance as it strongly correlates to worse patient survival (n=86 HR=2.2 p<0.01) using a panel of clinically annotated AML samples.
de Lima: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal