Key Points
Eight weekly transfusions of F-LR + pathogen-reduced donor platelets were accepted by 31 of 32 (97%) recipient dogs.
Among accepting recipients, none developed lymphocyte and only 2 had platelet antibodies not associated with platelet refractoriness.
Abstract
Human lymphocyte antigen alloimmunization to filter leukoreduced (F-LR) platelets occurs in about 18% of immunosuppressed thrombocytopenic hematology/oncology patients and represents a significant challenge for effective chemotherapy. In a dog platelet transfusion model, we have evaluated other methods of preventing alloimmune platelet refractoriness and demonstrated that successful methods in our dog model are transferable to man. In the present study, donor/recipient pairs were dog lymphocyte antigen DR-B incompatible (88% of the pairs), and recipient dogs received up to 8 weekly treated transfusions from a single donor (a highly immunogenic stimulus), or until platelet refractoriness. Continued acceptance of F-LR platelets occurred in 6 of 13 recipients (46%), but neither γ-irradiation (γ-I; 0 of 5) nor Mirasol pathogen reduction (MPR; 1 of 7) treatment of donor platelets prevented alloimmune platelet refractoriness. Combining γ-I with F-LR was associated with only 2 of 10 (20%) recipients accepting the transfused platelets. Surprisingly, F-LR platelets that then underwent MPR were accepted by 21 of 22 (95%) recipients (P < .001 vs F-LR + γ-I recipients). Furthermore, 7 of 21 (33%) of these accepting recipients demonstrated specific tolerance to 8 more weekly donor transfusions that had not been treated. In addition, platelet concentrates prepared from F-LR + MPR whole blood were also nonimmunogenic; that is, 10 of 10 (100%) recipients accepted donor platelets. Overall, 31 of 32 (97%) recipients accepted F-LR + MPR platelets; none developed antibodies to donor lymphocytes. These data are the highest rate of acceptance for platelet transfusions reported in either animals or man. This approach to platelet transfusion may be particularly important when supporting patients with intact immune systems, such as in myelodysplastic syndromes.
Introduction
One of the biggest problems in the transfusion support of chronically thrombocytopenic patients is alloimmunization to donor platelets and subsequent refractoriness to transfusion. Contaminating white blood cells (WBCs), rather than platelets themselves, may represent the major immunogen in transfused platelets. As demonstrated in the Trial to Reduce Alloimmunization to Platelets (TRAP trial), reduction of the probability of alloimmunization by more than half during a course of platelet transfusions can be achieved by reducing WBC contamination through filter leukoreduction (F-LR) or by UV-B irradiation (UVB).1 However, about 1 in 5 of the acute myelogenous leukemia patients in the TRAP trial still became refractory despite being immunosuppressed from their induction chemotherapy, and these rates were the same even in naïve patients who had never been transfused or pregnant.
We have successfully used a dog platelet transfusion model, as a preclinical approach, to predict which methods of modifying donor platelets would be successful in man,2-4 as have others using different animal models.5-7 Combining centrifuge leukoreduction (C-LR) with F-LR, regardless of which of 4 filters were used, 41 of 45 dogs (91%) accepted donor platelets without alloimmunization.4 These data suggested that more than one type of WBC is associated with alloimmunization, some being removed by filtration and others by centrifugation.8 However, it would be very difficult to quality control a C-LR process within routine blood center operations, thereby eliminating F-LR + C-LR as a practical approach to preventing alloimmunization.
The question then becomes what can be done to prevent immunization from the residual WBCs that are not removed by F-LR. We had previously tried combining F-LR with UVB in our dog model, and the benefits were additive but still resulted in 32% of the recipients becoming platelet refractory because of alloimmunization.2 Thinking UVB was perhaps not sufficiently effective in inactivating the residual WBCs, we assessed whether the combination of F-LR with γ-irradiation (γ-I) or Mirasol pathogen reduction (MPR; Terumo BCT, Lakewood, CO)9 might prevent platelet refractoriness because γ-I or MPR prevent transfusion-associated graft-versus-host disease (TAGVHD).9 We here report the effect of extending methods to prevent TAVGHD to preventing alloimmune platelet refractoriness.
Materials and methods
Experimental animals
Dogs were housed at the University of Washington Vivarium, and the experiments were approved by an animal use committee. Kennel-bred virgin mongrel hounds and beagles were used as donors and recipients, respectively (Marshall Bioresources, North Rose, NY, or Ridglan Farms, Mt Horeb, WI).
Preparation of donor platelets from PRP
Standard (STD).
Platelet-rich plasma (PRP) was prepared by centrifuging 30 to 60 mL of whole blood (WB), diluted 1:1 with Ringers-citrate-dextrose (RCD) for 15 min at 234g in a 150-mL transfer pack (Baxter/Fenwal, Deerfield, IL). The PRP was manually expressed into another transfer pack and centrifuged at 935g for 15 min; the supernatant was then expressed, and the pellet was resuspended in 6 mL of RCD.
Treated.
For F-LR, PRP (prepared as above) was F-LR with Pall PL1-B (pediatric) (Haemonetics, Braintree, MA) or Fenwal PLS-5A filters (East Hills, NY). For γ-I, PRP was γ-I at a dose of 25 Gy (GammaCell 1000 Irradiator; Atomic Energy of Canada, Mississauga, Ontario, Canada). For MPR, 5 mL or 3.5 mL of riboflavin were added to 50 mL of PRP or WB, respectively, and both were exposed to 100 J/mL in an illumination bag (150 mL ELP Illumination bag) using a Mirasol UV irradiation device.9 For combined treatments, the donor’s PRP or WB was subjected to more than 1 treatment.
Preparation of donor platelets from MPR WB
Two methods of assessing the immunogenicity of platelets prepared from MPR WB were performed: 1) WB was MPR as above, followed by PL1-B F-LR of the PRP prepared from the WB (MPR WB + F-LR PRP); or 2) filtration was performed on the WB using a platelet-sparing filter (IMUFLEX WB-SP, Terumo BCT),10 followed by MPR and then PRP preparation (F-LR WB + MPR).
Radiolabeling of donor platelets
Donor platelets were radiolabeled with 51chromium after completion of all treatments, resuspended in 6 mL of RCD, and 5 mL was injected within 4 to 8 h of collection.11 Blood samples were drawn 15-30 min after transfusion and daily for 3 days, and radioactivity was determined with a Wallac 1480 Wizard 3-inch γ Counter (Turku, Finland). Platelet survival was calculated using the weighted mean method and recoveries determined from the survival curve at 20 h.12
Platelet and WBC content of the platelet preparations
Automated platelet and WBC counts were done using an ABX Micros 60 hematology analyzer (Horiba Medical, Irvine, CA). The lower limit of sensitivity for the WBCs was 1 × 106 per transfusion.
Antibody detection
Baseline and weekly recipient serum samples were frozen at −80°C and batch-tested at study conclusion, along with antibody positive and negative sera, against both donor and autologous fresh platelets and lymphocytes. Flow cytometry was used to detect bound IgG using anti-dog IgG-fluorescein isothiocyanate (Jackson Immuno Research Laboratories, West Grove, PA).13 By gating on characteristic size and complexity, 10 000 lymphocyte and 20 000 platelet events were acquired and subsequently analyzed for FL-1 intensities for platelet-bound IgG, phycoerythrin binding for B-cell enumeration, and F1-4 binding for CD8 cell enumeration (FACScan, Lysis II; Becton-Dickinson, San Jose, CA). Using a 1024-channel scale, recipient sera with fluorescence that is ≥1.3 above autologous sera were considered antibody positive.
Dog lymphocyte antigen (DLA) DR-B typing
We developed an oligotyping assay that used allele discriminating oligonucleotide probes immobilized on nylon membranes to determine subject animals’ DLA DR-B types2,4,14-16 to assess the extent of major histocompatibility matching between donor and recipient animals. Whenever possible, we selected DR-B completely mismatched donors and recipients, because matching may be associated with high rates of tolerance.17-19
Trial design
Selection of donor/recipient pairs.
All donor and recipient dogs had normal radiolabeled autologous platelet recoveries and survivals before entering the allogeneic transfusion study. Baseline recipient sera were screened for antibodies against platelets and lymphocytes from a panel of 3 donor dogs. Donor/recipient pairs were selected as cross-match negative and, as often as possible, DR-B mismatched across both loci.
Evaluation of the immunogenicity of donor platelets.
Each recipient received up to 8 weekly transfusions of their assigned donor’s platelets or until they became platelet refractory. Platelet refractoriness was defined as 2 sequential 20-h posttransfusion platelet recoveries of ≤5%. The recipient was considered to have accepted platelets until the time of their first refractory transfusion.
Accepting recipients received up to 8 additional weekly STD (nontreated) transfusions from their same donor or until refractoriness to determine whether donor-specific tolerance had been induced.
Statistical methods
Estimates of rates of acceptance and tolerance and their associated confidence intervals were derived from the binomial distribution. Time to refractoriness was assessed using Kaplan-Meier curves and analyzed by log-rank test. Comparisons between treatments were analyzed using Fisher’s exact test. Comparisons of 20-h recoveries at different times within each group were evaluated by fitting linear mixed-effects models to recovery values with the recipient dog serving as the model’s random effect. P values associated with these statistics were adjusted for multiple comparisons using the Bonferroni method. Descriptive statistics are presented for cell contents from each of the platelet preparations.
Results
Platelet transfusions
Platelets averaged 1.0 to 2.7 × 109 per transfusion, and WBCs averaged 6.7 × 106 for STD transfusions and ≤1 × 106 for all F-LR transfusions.
Platelet acceptance rates
DLA-DRB matching.
The majority of donor-recipient pairs (52 of 59; 88%) were DLA-DRB mismatched at both loci, and the other 7 pairs shared 1 epitope. Epitope sharing did not increase acceptance of either treated or subsequent transfusions of STD platelets given to assess specific tolerance.
Single treatments.
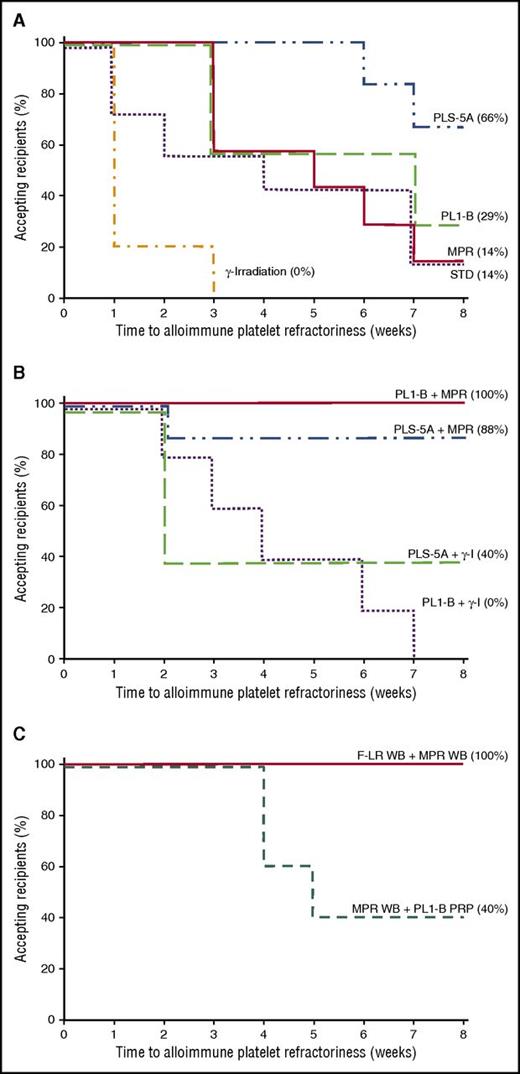
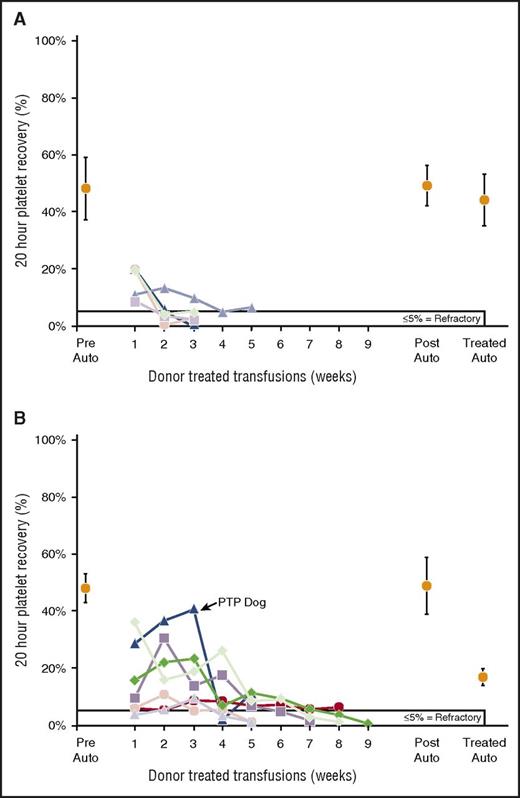
In our prior studies, 1 of 7 (14%), 2 of 7 (29%), and 4 of 6 (66%) recipients accepted STD,2 PL1-B F-LR,4 and PLS-5A F-LR4 platelets, respectively. In the present study, recipient acceptance rates for γ-I and MPR platelets were 0 of 5 and 1 of 7 (14%), respectively (supplemental Table 1, available on the Blood Web site). Small numbers precluded statistical comparisons. Time to refractoriness was significantly longer for PLS-5A F-LR platelets than for MPR and STD platelets (P ≤ .04) and longer for all platelets than for γ-I platelets (P < .04) (Figure 1A). Donor platelet recoveries were very poor for both γ-I and MPR transfusions (Figure 2A-B). However, the low recoveries for MPR platelets were at least partially due to the effects of MPR on platelets. Autologous platelet recoveries averaged 47% ± 5% and 21% ± 7% without and with MPR treatment, respectively (P < .001). Interestingly, 1 dog in the MPR group apparently developed posttransfusion purpura with a drop in platelet count from 261 000 per microliter at the end of the fourth MPR donor transfusion to 4000 per microliter after the fifth transfusion, and the dog was killed.
Duration of acceptance of single and combined treatments of donor platelet transfusions and donor platelets prepared from MPR-treated WB. (A) Number of weeks that recipient dogs accepted donor platelets that have not been treated (standard; dotted line), PLS-5A F-LR (dash-dot-dot line), PL1B F-LR (dashed line), MPR (solid line), or γ-irradiated (dashed-dotted line). Time to refractoriness was significantly longer for PLS-5A F-LR platelets than for STD and MPR platelets (P ≤ .04), and for all types of platelets than for γ-I platelets (P < .04). Of note, it was 3 weeks before any recipient became refractory to MPR and PL1-B F-LR platelets and 6 weeks to become refractory to PLS-5A F-LR platelets. In contrast, 80% of the recipients of γ-I transfusions became refractory after a single transfusion. Data for STD,2 PLS-5A,4 and PL1-B F-LR transfusions4 have been previously reported and are given here as reference. (B) Number of weeks that recipient dogs accepted donor platelets was significantly shorter for PL1-B F-LR + γ-I (dotted line) than for either PL1-B F-LR + MPR (solid line) or PLS-5A F-LR + MPR platelets (dash-dot-dot line) (P < .003), and it was significantly shorter for PLS-5A F-LR + γ-I platelets (dashed line) than for PL1-B F-LR + MPR platelets (P = .02) but not for PLS-5A F-LR + MPR platelets (P = .08). There was also no difference between PL1-B F-LR + γ-I and PLS-5A F-LR + γ-I transfusions (P = .48). (C) Number of weeks that recipient dogs accepted donor platelets that were prepared from MPR WB followed by PL1B F-LR of PRP (dashed line) was significantly shorter than than that for platelets prepared from F-LR WB followed by MPR of the WB (solid line) (P = .006). However, even MPR WB + PL1-B F-LR PRP platelets were accepted for at least 4 weeks.
Duration of acceptance of single and combined treatments of donor platelet transfusions and donor platelets prepared from MPR-treated WB. (A) Number of weeks that recipient dogs accepted donor platelets that have not been treated (standard; dotted line), PLS-5A F-LR (dash-dot-dot line), PL1B F-LR (dashed line), MPR (solid line), or γ-irradiated (dashed-dotted line). Time to refractoriness was significantly longer for PLS-5A F-LR platelets than for STD and MPR platelets (P ≤ .04), and for all types of platelets than for γ-I platelets (P < .04). Of note, it was 3 weeks before any recipient became refractory to MPR and PL1-B F-LR platelets and 6 weeks to become refractory to PLS-5A F-LR platelets. In contrast, 80% of the recipients of γ-I transfusions became refractory after a single transfusion. Data for STD,2 PLS-5A,4 and PL1-B F-LR transfusions4 have been previously reported and are given here as reference. (B) Number of weeks that recipient dogs accepted donor platelets was significantly shorter for PL1-B F-LR + γ-I (dotted line) than for either PL1-B F-LR + MPR (solid line) or PLS-5A F-LR + MPR platelets (dash-dot-dot line) (P < .003), and it was significantly shorter for PLS-5A F-LR + γ-I platelets (dashed line) than for PL1-B F-LR + MPR platelets (P = .02) but not for PLS-5A F-LR + MPR platelets (P = .08). There was also no difference between PL1-B F-LR + γ-I and PLS-5A F-LR + γ-I transfusions (P = .48). (C) Number of weeks that recipient dogs accepted donor platelets that were prepared from MPR WB followed by PL1B F-LR of PRP (dashed line) was significantly shorter than than that for platelets prepared from F-LR WB followed by MPR of the WB (solid line) (P = .006). However, even MPR WB + PL1-B F-LR PRP platelets were accepted for at least 4 weeks.
Twenty-hour donor platelet recoveries of single treatments of PRP. (A) All 5 recipients of γ-I platelets became platelet refractory with very poor recoveries even with their first donor transfusion. There were no differences between means of pretreatment (47% ± 11%) and posttreatment (49% ± 7%) autologous platelet recoveries (P = .66) nor between pretreated and treated (44% ± 9%) autologous platelet recoveries (P = .42). (B) Six of 7 recipients (86%) of MPR became refractory on treatment. One of these refractory dogs developed posttransfusion purpura after the fifth transfusion (her second refractory transfusion) when the recipient’s platelet count fell from 261 000 per microliter at the end of week 4 to 3000 per microliter the next week, and the dog was killed. The dog had high levels of antibodies to both her donor’s platelets (2.2 × autologous control sera) and lymphocytes (1.7 × autologous control sera). No autoantibodies were sought because the dog’s platelet count was too low. There were no differences between means of pretreatment (47% ± 5%) versus posttreatment (49% ± 11%) autologous platelet recoveries (P = .76), but there was a significant difference between pretreated and treated (21% ± 7%) autologous platelet recoveries (P < .001). PTP, posttransfusion purpura.
Twenty-hour donor platelet recoveries of single treatments of PRP. (A) All 5 recipients of γ-I platelets became platelet refractory with very poor recoveries even with their first donor transfusion. There were no differences between means of pretreatment (47% ± 11%) and posttreatment (49% ± 7%) autologous platelet recoveries (P = .66) nor between pretreated and treated (44% ± 9%) autologous platelet recoveries (P = .42). (B) Six of 7 recipients (86%) of MPR became refractory on treatment. One of these refractory dogs developed posttransfusion purpura after the fifth transfusion (her second refractory transfusion) when the recipient’s platelet count fell from 261 000 per microliter at the end of week 4 to 3000 per microliter the next week, and the dog was killed. The dog had high levels of antibodies to both her donor’s platelets (2.2 × autologous control sera) and lymphocytes (1.7 × autologous control sera). No autoantibodies were sought because the dog’s platelet count was too low. There were no differences between means of pretreatment (47% ± 5%) versus posttreatment (49% ± 11%) autologous platelet recoveries (P = .76), but there was a significant difference between pretreated and treated (21% ± 7%) autologous platelet recoveries (P < .001). PTP, posttransfusion purpura.
Combined treatments.
Platelets.
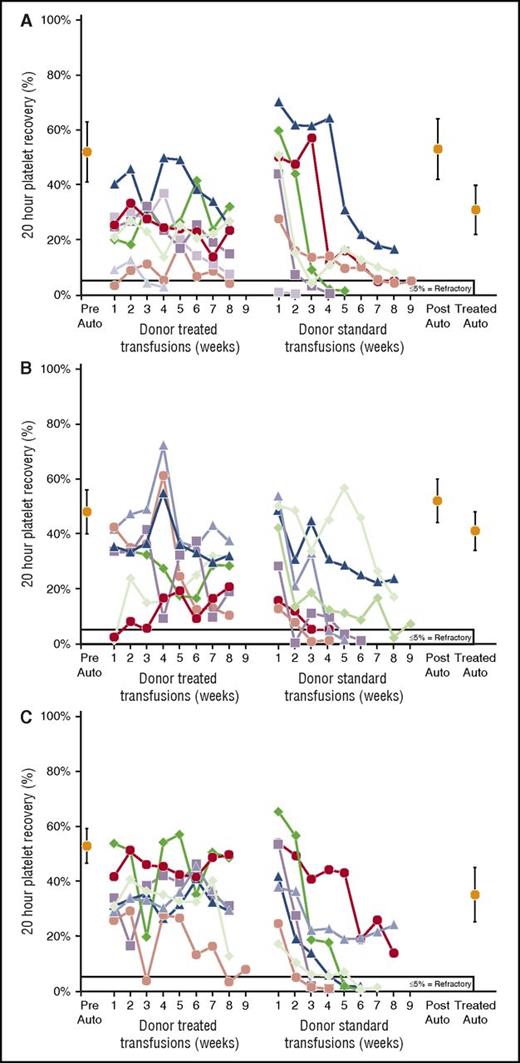
Because of the apparent limited ability of individual platelet treatments to prevent refractoriness, we combined F-LR using the PLS-5A or PL1-B filters with either γ–I or MPR. Adding γ-I reduced the acceptance of F-LR transfusions, but because of small numbers, the differences were not significant; that is, only 2 of 10 (20%) recipients accepted F-LR + γ-I platelets versus 6 of 13 (46%) recipients of F-LR platelets (P = .39) (supplemental Table 1). Recoveries of F-LR + γ-I platelets were very poor (Figure 3A-B). In contrast, 14 of 15 (93%) recipients accepted F-LR + MPR platelets (P < .001 in comparison with F-LR + γ-I). However, adding γ-I to F-LR + MPR platelets had no adverse effect; 7 of 7 recipients accepted the transfusions (supplemental Table 1). Overall, 21 of 22 recipients (95%) accepted F-LR + MPR platelets with no differences among the filters used. Time to refractoriness for PL1-B F-LR + γ-I platelets was significantly less than that for any F-LR + MPR platelets (P < .003) but not for PLS-5A F-LR + γ-I versus PLS-5A F-LR + MPR platelets (P = .08) (Figure 1B). Recoveries of F-LR + MPR or F-LR + MPR + γ-I donor platelets were consistently good (Figure 4A-C).
Twenty-hour donor platelet recoveries for platelets prepared from combined F-LR + γ-I treatments of PRP. (A) Two of 5 recipients (40%) accepted PLS-5A F-LR + γ-I platelets. There were no differences between means of pretreatment (43% ± 5%) and posttreatment (47% ± 2%) autologous platelet recoveries (P = .17) nor between pretreated and treated (49% ± 7%) autologous platelet recoveries (P = .51). (B) None of the 5 recipients accepted PL1-B F-LR + γ-I platelets. There were no differences between means of pretreatment (47% ± 6%) and posttreatment (51% ± 5%) autologous platelet recoveries (P = .19) nor between pretreated and treated (40% ± 20%) autologous platelet recoveries (P = .69).
Twenty-hour donor platelet recoveries for platelets prepared from combined F-LR + γ-I treatments of PRP. (A) Two of 5 recipients (40%) accepted PLS-5A F-LR + γ-I platelets. There were no differences between means of pretreatment (43% ± 5%) and posttreatment (47% ± 2%) autologous platelet recoveries (P = .17) nor between pretreated and treated (49% ± 7%) autologous platelet recoveries (P = .51). (B) None of the 5 recipients accepted PL1-B F-LR + γ-I platelets. There were no differences between means of pretreatment (47% ± 6%) and posttreatment (51% ± 5%) autologous platelet recoveries (P = .19) nor between pretreated and treated (40% ± 20%) autologous platelet recoveries (P = .69).
Twenty-hour donor platelet recoveries for platelets prepared from combined F-LR + MPR treatments of PRP. (A) Seven of 8 (88%) recipients of PLS-5A F-LR + MPR accepted treated transfusions and 2 of 7 (30%) accepting recipients also accepted subsequent standard platelet transfusions from their same donor. There was no difference between means of pretreatment (53% ± 12%) and posttreatment (57% ± 13%) autologous platelet recoveries (P = .52), but there was a significant difference between pretreated and treated (33% ± 9%) autologous platelet recoveries (P < .001). (B) All 7 recipients of PL1-B F-LR + MPR accepted treated donor transfusions, and 3 of 7 (43%) recipients also accepted subsequent standard transfusions from their same donor. There were no differences between means of pretreatment (46% ± 9%) and posttreatment (52% ± 9%) autologous platelet recoveries (P = .31), nor between pretreated and treated (43% ± 7%) autologous platelet recoveries (P = .56). (C) All 7 recipients of F-LR + MPR + γ-I accepted treated transfusions, and 2 of 7 (29%) also accepted subsequent standard transfusions from their same donor. Four of the donors’ platelets were PL1-B F-LR, and 3 were PLS-5A F-LR. There were no differences between means of pretreated (53% ± 6%) and treated (39% ± 7%) autologous platelet recoveries (P = .11).
Twenty-hour donor platelet recoveries for platelets prepared from combined F-LR + MPR treatments of PRP. (A) Seven of 8 (88%) recipients of PLS-5A F-LR + MPR accepted treated transfusions and 2 of 7 (30%) accepting recipients also accepted subsequent standard platelet transfusions from their same donor. There was no difference between means of pretreatment (53% ± 12%) and posttreatment (57% ± 13%) autologous platelet recoveries (P = .52), but there was a significant difference between pretreated and treated (33% ± 9%) autologous platelet recoveries (P < .001). (B) All 7 recipients of PL1-B F-LR + MPR accepted treated donor transfusions, and 3 of 7 (43%) recipients also accepted subsequent standard transfusions from their same donor. There were no differences between means of pretreatment (46% ± 9%) and posttreatment (52% ± 9%) autologous platelet recoveries (P = .31), nor between pretreated and treated (43% ± 7%) autologous platelet recoveries (P = .56). (C) All 7 recipients of F-LR + MPR + γ-I accepted treated transfusions, and 2 of 7 (29%) also accepted subsequent standard transfusions from their same donor. Four of the donors’ platelets were PL1-B F-LR, and 3 were PLS-5A F-LR. There were no differences between means of pretreated (53% ± 6%) and treated (39% ± 7%) autologous platelet recoveries (P = .11).
WB.
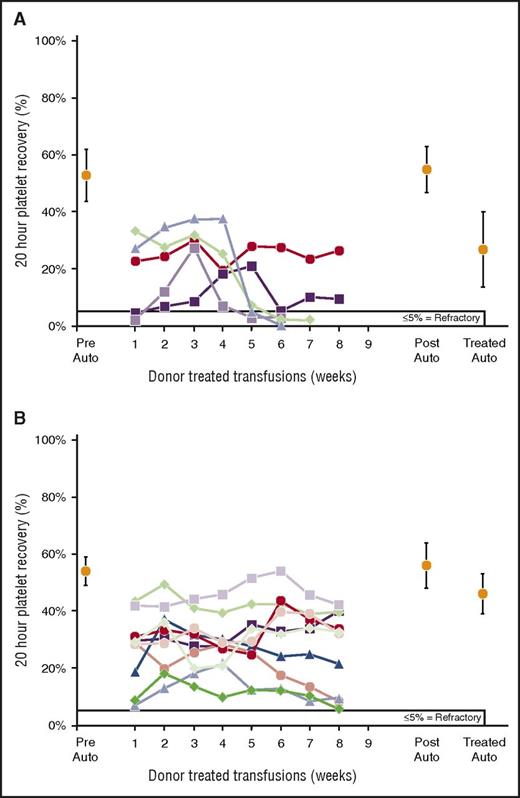
MPR of WB followed by PL1B filtration of the PRP prepared from the WB (MPR WB + F-LR PRP) resulted in 2 of 5 (40%) accepting recipients (supplemental Table 1) versus 10 of 10 (100%) accepting recipients if the WB was first F-LR followed by MPR of the WB (F-LR WB + MPR; P < .01). Time to refractoriness was significantly longer for F-LR WB + MPR than for MPR WB + F-LR PRP (P = .006), although the latter platelets were accepted for at least 4 weeks (Figure 1C). Recoveries of F-LR + MPR WB-derived platelets (Figure 5B) were very similar to those of F-LR + MPR PRP platelets (Figure 4A-C).
Twenty-hour donor platelet recoveries for platelets prepared from MPR WB. (A) MPR WB followed by PL1-B F-LR of PRP. Two of 5 recipients accepted treated transfusions. There were no differences between means of pretreatment (53% ± 9%) and posttreatment (55% ± 8%) autologous platelet recoveries (P = .83), but there was a significant difference in means of pretreated and treated (27% ± 13%) autologous platelet recoveries (P = .002). (B) F-LR of WB followed by MPR of WB. All 10 recipients accepted treated donor platelets. There were no differences between means of prestudy (54% ± 5%) and poststudy (56% ± 8%) autologous platelet recoveries (P = .78), but there was a significant difference between pretreated and treated (46% ± 7%) autologous platelet recoveries (P = .03).
Twenty-hour donor platelet recoveries for platelets prepared from MPR WB. (A) MPR WB followed by PL1-B F-LR of PRP. Two of 5 recipients accepted treated transfusions. There were no differences between means of pretreatment (53% ± 9%) and posttreatment (55% ± 8%) autologous platelet recoveries (P = .83), but there was a significant difference in means of pretreated and treated (27% ± 13%) autologous platelet recoveries (P = .002). (B) F-LR of WB followed by MPR of WB. All 10 recipients accepted treated donor platelets. There were no differences between means of prestudy (54% ± 5%) and poststudy (56% ± 8%) autologous platelet recoveries (P = .78), but there was a significant difference between pretreated and treated (46% ± 7%) autologous platelet recoveries (P = .03).
Tolerance induction
Too few recipients accepted γ-I, MPR, and F-LR + γ-I platelets to assess the impact of the treatments on the induction of tolerance of subsequent untreated (STD) platelets. For recipients who accepted F-LR + MPR platelets, 7 of 21 (33%) accepted STD transfusions from their same donor (Figure 4A-C; supplemental Table 1). The acceptance rates of STD platelets were the same for both filters when used with or without additional γ-I.
Antibody results
Following MPR or γ-I platelet transfusions, 11 of 12 (92%) recipients became platelet refractory, and of the 10 refractory animals tested, 8 developed antibodies to donor lymphocytes, 7 developed antibodies to platelets, and 2 were antibody negative (supplemental Table 2). The one MPR-accepting recipient had both donor lymphocyte and platelet antibodies. One MPR recipient developed posttransfusion purpura after the fifth transfusion with very high antibodies to both the donor’s platelets (2.2× autologous control sera) and lymphocytes (1.7× autologous control sera) (Figure 2B).
Of the 31 recipients who accepted their donor’s F-LR + MPR platelets prepared from either PRP or WB, 30 were tested for antibodies; none had antibodies to donor lymphocytes, and only 2 had platelet antibodies (supplemental Table 2).
Of the 12 recipients who were refractory to combined treated-donor transfusions, 10 were tested for antibodies, and 6 had antibodies to donor lymphocytes, 4 had antibodies to donor platelets, and 3 were negative.
Of the 7 recipients who had developed tolerance to their donor’s STD platelets, 3 had antibodies to both their donor’s lymphocytes and platelets and 4 were antibody negative (supplemental Table 2). Of the 14 recipients who rejected STD platelets, 13 were antibody tested, and 12 developed antibodies to their donor’s lymphocytes, 3 developed antibodies to platelets, and 1 was antibody negative.
Overall, the antibody results correlated with refractoriness to their donor’s platelets with a sensitivity of 82%, specificity of 76%, positive predictive value of 85%, and negative predictive value of 73%.
Radiolabeled autologous and donor platelet recovery and survival data
All 59 recipient dogs had baseline radiolabeled autologous platelet recoveries and survivals, averaging 48% ± 7% and 5.1% ± 1.2 days, respectively (supplemental Table 3). Forty recipients (68%) had repeat poststudy recoveries of 51% ± 8% (106% of their baseline data) and survivals of 5.0% ± 0.8 days (97% of their baseline data). These autologous data indicate that the recipients were clinically stable; therefore, any donor refractoriness was considered alloimmune, even if no antibodies were detected.
There were no adverse effects of γ-I on either autologous platelet recoveries or survivals when it was used alone or when added to F-LR (supplemental Table 3). In contrast, MPR used alone reduced both autologous platelet recoveries and survivals to 45% and 37% of their baseline values, respectively. However, when platelets were PL1-B F-LR or PLS-5A F-LR followed by MPR, recoveries improved to 94% and 62%, and survivals improved to 40% and 33% of their baseline values, respectively. Furthermore, when WB was F-LR + MPR, autologous platelet recoveries were 85%, and survivals were 91% of baseline values. These data clearly demonstrate the benefits of preceding MPR with F-LR.
Data were also analyzed on the posttransfusion recoveries and survivals of the donor’s platelets from the first transfusion until refractoriness (supplemental Table 3). Even the first posttransfusion donor platelet recoveries, as a percentage of the recipient’s autologous recoveries, were so low for γ-I (34%), MPR (32%), and PL1-B + γ-I (17%) transfusions that platelet survivals could not be determined. For PLS-5A + γ-I platelets, first donor platelet recoveries averaged 60%, and survivals averaged 87% of their recipient’s autologous data, respectively, and these values for subsequent transfusions until refractoriness averaged 67% and 65%, respectively.
Interestingly, first donor platelet recoveries were better for F-LR + MPR + γ-I transfusions than for F-LR + MPR transfusions without γ-I (82% vs. 49% of autologous recipients’ baseline data, respectively; P = .03), and this difference continued for 8 weeks (87% vs 53%, respectively; P = .02). Similarly, there were also differences in platelet survivals between the F-LR + MPR + γ-I and the F-LR + MPR donor transfusions, that is, 46% versus 35% of autologous recipients’ baseline data, respectively (P = .07) for first donor survivals and 47% versus 35% of autologous recipients’ baseline data until refractoriness, respectively (P = .007).
For MPR WB, first donor platelet recoveries, as a percentage of autologous baseline recoveries, were not statistically significantly different for F-LR WB + MPR and MPR WB + F-LR PRP (49% vs 34%, respectively; P = .32). This was also the case for platelet survivals (89% vs 40%, respectively; P = .16); these patterns continued until refractoriness.
Discussion
Our current studies have conclusively demonstrated that F-LR followed by MPR eliminates the immunogenicity of both WBCs and platelets in our dog platelet transfusion model. The combined F-LR + MPR approach is likely successful because F-LR removes most of the immunizing WBCs, and the residual immunizing WBCs are rendered nonfunctional by MPR. Exposure to UV light in the presence of riboflavin during the MPR process causes irreparable damage to nucleic acids inhibiting white cell replication and their function as immune stimulators. Furthermore, we expect these results will be transferable to man as demonstrated by our prior dog studies in which the benefits of F-LR and UV-B irradiation of donor platelets in our dog model were shown to also prevent alloimmunization to donor platelets in the TRAP trial.1 In fact, these approaches were even more successful in the TRAP trial than in our dog model perhaps because the patients were immunosuppressed versus the dogs’ normal immune system.
We chose recipient acceptance of donor platelets as our primary endpoint for two reasons: 1) clinical relevance; and 2) lack of absolute correlation between alloimmunization and clinical refractoriness. Our previous dog studies demonstrated that recipient acceptance rates of F-LR platelets varied from 29% to 66%, depending on the filter used.4 Our current study showed acceptance rates of donor platelets treated with either γ-I or MPR to inactivate contaminating WBCs were successful in only 0% and 14% of transfusion pairs, respectively (supplemental Table 1).
F-LR + MPR platelets were accepted by 14 of 15 recipients (93%) in comparison with acceptance of F-LR + γ-I platelets in 2 of 10 recipients (P < .001) (supplemental Table 1), and time to refractoriness was also significantly longer (P < .001) (Figure 1B). Although MPR prevented TAGVHD in an animal model,20 some physicians may not accept MPR and γ-I as clinically equivalent in preventing TAGVHD. Therefore, γ-I was added to F-LR + MPR treatment of platelets, and 7 of 7 dogs accepted this treatment, indicating that this additional treatment did not abrogate the combined benefit of the first 2.
Overall, 21 of 22 (95%) dogs who received some type of F-LR + MPR donor platelets accepted these transfusions. Because our studies with PRP were performed only by filtering the PRP followed by MPR treatment, we do not know whether the combined F-LR + MPR treatments would be effective if performed in the reverse order. Donor-specific tolerance to STD platelets from their same donors was induced in 7 of 21 (33%) recipients who accepted F-LR + MPR platelets with no apparent differences in acceptance based on the filter used with or without γ–I (supplemental Table 1).
Treatment of WB generated very unexpected results. We first tested MPR of the WB followed by F-LR of the PRP because there are many platelet leukoreduction filters but only 1 US Food and Drug Administration–licensed platelet-sparing WB leukoreduction filter. Unfortunately, only 40% of recipients accepted platelets when MPR preceded F-LR (supplemental Table 1). Conversely, when WB was F-LR followed by MPR of the WB, donor platelets prepared from the treated WB were accepted by 100% of recipients. These data may suggest that the majority of the immunogenic white cells must be removed by filtration before MPR can inactivate the remaining immunogenic white cells.
Of the 32 recipients who received F-LR WB or PRP that was then MPR, 31 (97%) accepted. Thirty accepting recipients were antibody tested, and none developed antibodies to their donor’s lymphocytes (supplemental Table 2). However, we have always been concerned that even with abrogation of WBC immunization, platelets themselves would be immunogenic as they express human lymphocyte antigen, platelet-specific antigens, and ABO antigens.21-23 Surprisingly, only 2 (7%) of the accepting recipients developed platelet antibodies, and these antibodies did not cause platelet refractoriness. These results are consistent with the TRAP trial data for which platelet antibodies were uncommon (6% to 11%), did not vary by treatment group (treated or control), and were not associated with platelet refractoriness.1 This almost uniform acceptance of F-LR + MPR platelets occurred in spite of 8 weekly exposures to the same donor’s antigens (a highly immunogenic stimulus with mongrel hound donors and female beagle recipients) and a high degree of DLA DR-B mismatching pairs (88% of donor/recipient pairs). We also used 2 leukoreduction filters that produced different outcomes when used alone but not when combined with MPR, suggesting that although residual WBC contents may differ among filters,6,21,24 any remaining WBCs can be inactivated by MPR (Figure 1A-B; supplemental Table 1).
There was no effect of γ-I on dog autologous platelet recoveries or survivals, similar to observations in humans (Figure 2B; supplemental Table 3).25 However, recoveries of even the first γ-I donor transfusions were markedly reduced and continued to decrease until refractoriness (Figure 2B), similar to the effects of γ-I on donor platelets in TRAP trial patients1 for whom platelet increments were significantly decreased and refractoriness was significantly increased.26 Adding γ–I to F-LR (ie, F-LR + γ–I) platelets reduced acceptance rates (supplemental Tables 1 and 3), and this observation is consistent with results from a cardiopulmonary bypass patient transfusion study in which γ-I of F-LR blood products abrogated the benefits of F-LR in preventing alloimmunization.27 If γ-I enhances alloimmunization to F-LR platelets, this may be another reason to incorporate MPR into clinical practice. If MPR alone is not judged sufficiently protective against TAGVHD, γ-I can be added to F-LR + MPR platelets without increasing platelet alloimmunization rates.
Mirasol treatment reduces both autologous platelet recoveries and survivals to 45% and 37% of their baseline values, respectively (Figure 2B; supplemental Table 3). Mirasol has been previously reported to reduce human autologous radiolabeled platelet viability by 25%.28 However, adding F-LR to MPR (ie, F-LR + MPR) improved both autologous and donor platelet recoveries and survivals (Figure 4A-B; supplemental Table 3). When WB was F-LR followed by MPR, platelets prepared from the WB had recoveries and survivals that were 85% and 91%, respectively, of baseline values (Figure 5B; supplemental Table 3). The treated donor platelet recoveries and survivals mirrored the autologous data. It is not surprising that MPR of the WB was less injurious to platelets than was MPR of platelets because the RBCs would have adsorbed some of the UV light of the MPR treatment.
Because of the high acceptance rates of both F-LR + MPR platelets and platelets prepared from F-LR WB + MPR with no evidence of lymphocyte antibodies and only 2 recipients with platelet antibodies not associated with platelet refractoriness, it is possible that this approach will be effective in both immunosuppressed as well as nonimmunosuppressed patients, for example, those with aplastic anemia or myelodysplastic syndromes. F-LR + MPR of red cells might also prevent alloimmunization to these cells, similarly to the success seen in platelet transfusions. F-LR is routinely incorporated into blood center practice, and adding MPR could easily be accomplished. Unfortunately, MPR of platelets is not licensed in the United States, but it is in Europe. Licensing studies for MPR of platelets in the United States are planned for 2017. The demonstration that these combined F-LR and MPR methods are effective with both platelets prepared from PRP and WB suggests that they can be used for both apheresis platelets and platelet concentrates.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Many thanks to both Suzanne Marschner and Ray Goodrich at Terumo for their support and technical help with incorporating MPR into this project. Ginny Knight was invaluable in the preparation of the manuscript. Thanks also to student laboratory assistants Willy Cheung, Michael Shapiro, and Kristina Tkachenko for their assistance in performing some of the studies and data compilation.
All filters were generously donated by the Pall Corporation (East Hills, NY) or Haemonetics Corporation (Braintree, MA). The Mirasol pathogen reduction technology was kindly supplied by the Terumo Corporation.
This research study was supported by a grant from the U.S. Army Medical Research and Materiel Command, US Department of Defense (Contract W81XWH-07-1-0578) and in part by a grant provided by Terumo BCT, Lakewood, CO.
The authors certify that neither they nor their institution have an affiliation or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript. No company was involved in the design or execution of the studies.
Authorship
Contribution: S.J.S. designed the research, analyzed and interpreted data, and wrote the manuscript; E.P. performed the research, collected data, and analyzed and interpreted data; S.L.B. performed the research, collected data, analyzed and interpreted data, and performed statistical analyses; T.C. and I.G. performed the research, collected data, and analyzed and interpreted data; L.G., Y.L., and K.N. designed the research and analyzed and interpreted data; and D.B. performed statistical analyses and wrote the manuscript.
Conflict-of-interest disclosure: S.J.S. has received research support from Terumo BCT and is a consultant. S.L.B. is a consultant to Terumo BCT. The remaining authors declare no competing financial interests.
Correspondence: Sherrill J. Slichter, Platelet Transfusion Research, Bloodworks Northwest, 921 Terry Ave, Seattle, WA 98104-1256; e-mail: sherrills@bloodworksnw.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal