In this issue of Blood, Jäckel et al define an unexpected role for the microbiome in regulating murine plasma von Willebrand factor (VWF) levels. In particular, commensal gut microbiota are shown to regulate VWF synthesis in liver sinusoidal endothelial cells through a Toll-like receptor-2 (TLR2)–dependent pathway. Collectively, these novel findings provide important insights into the biological mechanisms through which commensal microbiota may modulate cardiovascular pathology.1
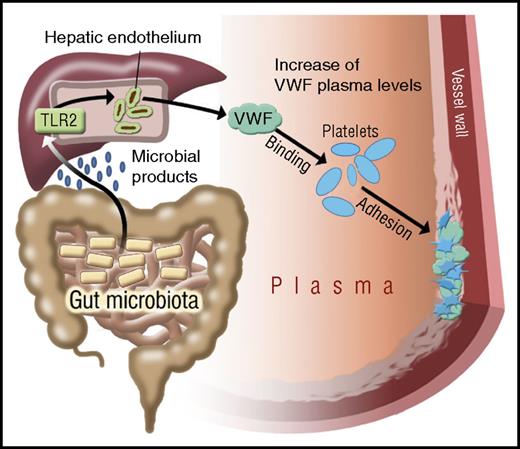
Pathway through which gut microbiota modulate a prothrombotic effect. Gut microbial–derived products pass through the portal circulation to the liver, where they stimulate TLR2 signaling in hepatic sinusoidal endothelial cells. This signaling results in enhanced VWF synthesis and secretion. The consequent significant increase in plasma VWF levels enhances platelet accumulation at sites of arterial injury. See Figure 7 in the article by Jäckel et al that begins on page 542.
Pathway through which gut microbiota modulate a prothrombotic effect. Gut microbial–derived products pass through the portal circulation to the liver, where they stimulate TLR2 signaling in hepatic sinusoidal endothelial cells. This signaling results in enhanced VWF synthesis and secretion. The consequent significant increase in plasma VWF levels enhances platelet accumulation at sites of arterial injury. See Figure 7 in the article by Jäckel et al that begins on page 542.
In the normal population, plasma VWF levels demonstrate significant interindividual variation. This variation in VWF levels has direct translational significance. Elevated levels of the VWF–factor VIII complex are associated with increased risk of myocardial infarction, stroke, and venous thromboembolism.2,3 Conversely, deficiency of VWF is responsible for the most commonly inherited bleeding disorder, von Willebrand disease (VWD). Previous twin studies estimated that 66% of the variation in plasma VWF:antigen (Ag) levels is genetically determined.4 Among these genetic determinants, the ABO blood group is of particular importance, such that group O individuals have plasma VWF levels 30% lower compared with non-O individuals.5 In addition, a number of environmental determinants have also been shown to influence the wide variation in VWF levels.4 For example, significant increases in plasma VWF levels are associated with increasing age, exercise, stress, pregnancy, surgery, and any other causes of an acute-phase response (eg, chronic inflammation or malignancy).
Emerging recent data suggest that symbiotic gut microbiota may be important in determining cardiovascular disease risk.6,7 However, the molecular mechanisms underpinning this association remain poorly defined; gut microbiota–derived metabolites have been shown to directly influence platelet function.8 Jäckel et al describe an exciting new pathway directly linking gut microbiota to thrombotic risk by demonstrating that these commensals also constitute a previously unrecognized environmental determinant of plasma VWF levels. Thus, plasma VWF:Ag levels were ∼30% lower in germ-free (GF) mice compared with conventionally raised mice. Importantly, despite the fact that VWF levels vary significantly between different murine strains, this effect of gut microbiota on plasma VWF was consistent across different strains. In striking contrast, however, neither plasma VWF multimer distribution nor ADAMTS13 levels were affected by the presence or absence of gut microbiota.
Although intestinal gut microbes do not typically access the liver under noninfectious conditions, previous studies have shown that gut microbial–derived products constantly leak into the tissues and portal circulation. Moreover, pathogen-associated molecular patterns derived from these microbiota can trigger TLR signaling in the host.9 Jäckel et al demonstrate that the increased plasma VWF levels in the presence of a normal gut microbiota are primarily attributable to enhanced VWF expression in liver sinusoidal endothelial cells. Furthermore, the effect of commensal gut microbial flora in regulating hepatic expression of VWF is modulated through a TLR2-dependent pathway (see figure). Importantly, this effect of the gut microbiome on plasma VWF levels translates into significant differences in thrombus formation in a murine carotid artery ligation model of arterial thrombosis. Thus, in keeping with their reduced plasma VWF levels, platelet accumulation at the site of vascular injury was significantly attenuated in mice raised in GF conditions. Cumulatively therefore, as illustrated, these data establish a potential new pathogenic mechanism through which gut microbiota can significantly enhance thrombotic risk in a VWF-dependent manner.
Further studies will clearly be required to investigate whether human gut commensal bacterial species modulate plasma VWF levels in a similar manner and to define the magnitude of any such effects. Moreover, it remains unclear whether more subtle qualitative and quantitative changes in human gut microbiota expression (eg, with increasing age or following specific antibiotic therapies) may also serve to regulate VWF expression in liver sinusoidal endothelial cells. Additional studies will also be necessary to determine whether any effects of human gut commensals upon hepatic VWF synthesis are exclusively mediated via TLR2-dependent signaling, or whether alternate microbial pattern-recognition receptor pathways also contribute to induction of VWF synthesis. Addressing these important questions in humans will require carefully designed studies in order to distinguish between increased hepatic synthesis as opposed to endothelial cell activation and regulated VWF secretion from Weibel-Palade body stores. Of note, previous studies have demonstrated that TLR2 signaling can also induce Weibel-Palade body exocytosis.10
Given the enormous morbidity and mortality associated with cardiovascular disease, the findings of Jäckel et al are not only of intrinsic scientific importance, but also of direct clinical significance. Further elucidation of the molecular mechanisms through which products derived from gut microbiota lead to the generation of a prothrombotic milieu may offer opportunities to develop novel targeted therapeutic strategies to reduce the risk of cardiovascular disease. Specifically, identification of the particular bacterial species responsible for this effect will aid not only in deciphering the microbial-sensing pathways that promote increased VWF synthesis, but also point to probiotic or antibiotic strategies that might be used to actively modulate VWF levels. These data also raise the tantalizing possibility that fluctuations in an individual’s VWF level induced by environment, disease, or age may be at least in part determined by their gut flora composition. In addition, the study also raises important questions regarding the potential importance of gut microbiota in patients with a number of other conditions, including type 1 VWD and thrombotic thrombocytopenic purpura. Finally, given our evolving understanding of how gut microbiome composition contributes to the instruction and maintenance of the immune system, and the increased role for immunity in regulating clot development, this study is likely to represent a “starting gun” for further studies designed to delineate additional unrecognized connections between gut microbiota and other facets of the hemostatic system.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal