In this issue of Blood, Rodrigues et al use a conditional knockout mouse model to describe a novel role for p53 in the differentiation and maintenance of medullary thymic epithelial cells (mTECs), tissue-restricted antigen (TRA) expression, and self-tolerance.1
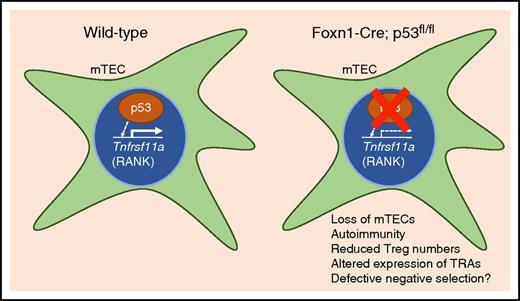
p53 is expressed in mTECS and promotes the expression of Tnfrsf11a (RANK). Conditional knockout of p53 in TECs results in reduced expression of RANK, a reduction in the overall number of mTECs, tissue-specific autoimmunity, and altered expression of TRAs. p53 is a critical modulator of mTEC homeostasis and the generation of a self-tolerant T-cell repertoire.
p53 is expressed in mTECS and promotes the expression of Tnfrsf11a (RANK). Conditional knockout of p53 in TECs results in reduced expression of RANK, a reduction in the overall number of mTECs, tissue-specific autoimmunity, and altered expression of TRAs. p53 is a critical modulator of mTEC homeostasis and the generation of a self-tolerant T-cell repertoire.
The thymus is essential for the generation of mature, self-tolerant T cells. Within the thymus, maturing T cells interact with 2 distinct epithelial cell types. Cortical thymic epithelial cells (cTECs) mediate positive selection, and mTECs establish self-tolerance. For this reason, mTECs promiscuously transcribe TRAs whose expression is otherwise restricted to differentiated organs. The presentation of such self-antigens to maturing T cells results in the deletion of autoreactive T cells and also guides the development of regulatory T cells (Tregs). The transcription factors controlling the expression of TRAs in mTECs therefore play an essential role in generating the self-tolerant T-cell repertoire, but our knowledge of these factors is incomplete. Aire and Fezf2 are 2 transcriptional controllers known to be required for the expression of many TRAs in mTECs, but not all TRA expression is controlled by these 2 factors, suggesting the existence of additional, currently unidentified factors necessary for the expression of TRAs.2,3
Rodrigues et al investigated p53 function in TECs by crossing p53 conditional knockout (p53cKO) mice with Foxn1-Cre mice to disrupt p53 function in both cTEC and mTEC populations (see figure).1 Most healthy cells contain low levels of p53 protein, which is continuously degraded by the proteasome. p53 is stabilized in response to many different cellular stresses, including genomic damage, oncogene activation, ribosomal stress, and loss of cell-matrix adhesion.4 When stabilized, p53 induces transcription of many genes to regulate processes such as cell cycle arrest, DNA repair, apoptosis, and control of mitochondrial respiration.4 The authors found that postnatal p53cKO mice showed a significant reduction in the number of mTECs, but the cTEC compartment was only minimally affected.
Interestingly, the authors observed reduced expression of Tnfrsf11a (receptor activator of nuclear factor κB [RANK]) in p53cKO mTECs. RANK binds RANK ligand (RANKL), which is produced by T cells, and RANKL/RANK signaling is essential for the growth and maturation of Aire-expressing mTECs.5 The authors identified p53 response elements upstream of the Tnfrsf11a promoter and demonstrated that p53 is capable of inducing expression from those sites by induction of luciferase in p53-deficient mouse embryonic fibroblasts (MEFs).6 Taken together, these results suggest that one function of p53 is to guide the maturation of Aire-expressing mTECs via expression of RANK.
The authors investigated additional functions of p53 by transcriptionally profiling cTECs and mTECs from wild-type and p53cKO animals. Surprisingly, they identified thousands of differentially expressed genes in p53-deficient mTECs, many of which were TRAs. Levels of Aire and Fezf2 were unchanged in adult p53cKO mTECs, although expression of Aire was developmentally delayed. Many TRAs with differential expression in p53cKO mTECs are known to be expressed independently of Aire. The results reveal a previously unappreciated role for p53 in the expression of many TRAs that appears distinct from known functions of Aire and Fezf2.
The loss of TRA expression is predicted to result in autoimmunity, and indeed, the authors report autoimmune defects in p53cKO animals, including increased infiltration of lymphocytes into salivary and lacrimal glands resulting in inflammatory lesions. One potential cause of autoimmunity is a reduction in the number or function of Tregs, and the authors found a significant reduction in the number of CD25+Foxp3+ Tregs in the p53cKO thymus. Importantly, the authors showed that when p53cKO-derived thymocytes were transferred to T-cell deficient mice, the mice developed autoimmune symptoms, including diarrhea and accelerated weight loss. Thus, T cells educated by p53-deficient mTECs are not appropriately self-tolerant.
It is unclear whether and how these newly identified functions of p53 in mTECs align with the well-established roles it plays in response to cellular stress. Past work indicates that Aire in mTECs associates with proteins implicated in the DNA damage response, including Ku80, DNA-PKcs, γH2AX, and PARP-1.7 One possibility is that mechanisms driving transcription of otherwise repressed genes in mTECs result in DNA damage and so stabilize p53 protein in a subpopulation of mTECs. An alternative possibility derives from the observation that p53 can function as a transcriptional activator in cells without stress.8 An intriguing possibility is that some transcription factors, including p53, may promote the expression of the same gene targets in mTECs as they do in other tissues. The absence of a given transcription factor specifically in mTECs would result in altered expression of its respective target genes in mTECs, but not other tissues, resulting in tissue-specific autoimmunity. A prediction of this model is that mice that lack activity of the transcription factor in all tissues would not display such autoimmune symptoms. Indeed, the autoimmune phenotypes reported here for mice lacking p53 in TECs have not been previously reported in mice with germ line deficiency of p53.9 Whatever the mechanism, it will be fascinating to determine how this versatile transcription factor contributes to self-tolerance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal