In this issue of Blood, Yoshimi et al describe a simple method to establish patient-derived xenografts (PDXs) of chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia (JMML) with remarkable efficiency. These morphologically, immunophenotypically, and genetically accurate models will pave the way for in vivo biological studies and preclinical testing of novel therapies.1
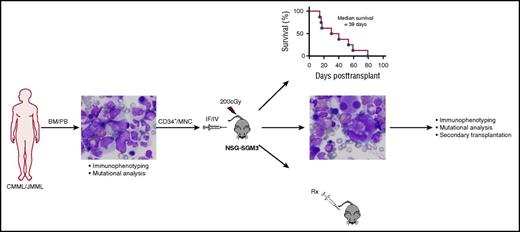
Establishment of a robust PDX model of CMML and JMML. Experimental schema employed by Yoshimi et al to establish and characterize the PDX model of CMML and JMML. Well-annotated primary patient samples from either the bone marrow (BM) or peripheral blood (PB) of CMML and JMML patients were used. Both human CD34-enriched cells (CD34+) and unfractionated mononuclear cells (MNCs) engrafted sublethally irradiated NSG-SGM3 mice after intrafemoral (IF) or IV injection. Survival and extensive analyses of the xenografted disease was performed. Secondary transplantation and in vivo drug (Rx) testing was performed. Path images courtesy of Andrea Marcogliese, Texas Children’s Hospital. The figure has been adapted from Figure 1 of Yoshimi et al that begins on page 397.
Establishment of a robust PDX model of CMML and JMML. Experimental schema employed by Yoshimi et al to establish and characterize the PDX model of CMML and JMML. Well-annotated primary patient samples from either the bone marrow (BM) or peripheral blood (PB) of CMML and JMML patients were used. Both human CD34-enriched cells (CD34+) and unfractionated mononuclear cells (MNCs) engrafted sublethally irradiated NSG-SGM3 mice after intrafemoral (IF) or IV injection. Survival and extensive analyses of the xenografted disease was performed. Secondary transplantation and in vivo drug (Rx) testing was performed. Path images courtesy of Andrea Marcogliese, Texas Children’s Hospital. The figure has been adapted from Figure 1 of Yoshimi et al that begins on page 397.
JMML and CMML are poor prognosis myeloproliferative/myelodysplastic overlap syndromes that pose significant therapeutic challenges to pediatric and adult oncologists, respectively. Currently, hematopoietic stem cell transplant (HSCT) is the only proven curative therapy for both. Still, HSCT cures only ∼50% of patients with JMML, and it is not a viable option for the majority of CMML patients, given their advanced age and comorbitities.2,3 A paucity of experimental systems that fully recapitulate features of these diseases represents a substantial barrier to the development of novel therapies.
Xenograft models of human acute leukemias that use ever-improving strains of immunocompromised mice have allowed for seminal investigations, including the identification and characterization of leukemia stem cells and preclinical testing of novel therapies.4 However, xenograft models for nonacute leukemias have lagged behind, owing to inefficient engraftment in most of the immunodeficient murine strains used thus far. In their article, Yoshimi et al demonstrated the great capacity of human CMML and JMML samples to engraft immunocompromised mice with transgenic expression of human stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-3 in the NOD/SCID-Il2rγcnull background (NSG-SGM3) (see figure). Efficient engraftment was achieved with relatively low cell numbers independent of the cell source (bone marrow vs peripheral blood), population (CD34-enriched vs unfractionated MNCs), and mode of injection (intrafemoral vs IV). Importantly, these xenografts closely phenocopied clinical disease features and genetic characteristics of the primary patient samples and enabled in vivo drug sensitivity testing, proving to be a valuable new tool for the study of these challenging diseases.
The simplicity of the model reported by Yoshimi et al makes it highly appealing compared with previously published models. Several other groups have reported attempts to establish xenograft models of CMML and JMML by using a number of different immunodeficient mouse strains with and without the addition of human myeloid supporting cytokines and with varying cell doses and sample sources.5-7 Each of these strategies achieved some success, and we lack a direct comparison of the models. Nevertheless, these studies together suggest that severe adaptive and innate immunodeficiency combined with expression of human myeloid supporting cytokines is superior to either strategy alone. Similarly, the greatest engraftment of primary human acute myeloid leukemia samples was achieved in NSG-SGM3 mice followed by NOD/SCID-SGM3, NSG, and NOD/SCID.8
A number of mouse models genetically engineered to harbor mutations common to CMML and JMML have provided important mechanistic insights into disease pathogenesis.2,3 However, these models lack the tumor heterogeneity inherent to human hematological malignancies. The capacity of xenograft models to recapitulate this heterogeneity opens the door for investigations of clonal hierarchy, including the potential to identify, functionally characterize, and define the underlying molecular drivers of the elusive disease-initiating cells. The utility of the xenotranplant model for this purpose is contingent on the xenografted disease accurately reflecting the clonal composition of the human disease from which it was derived. Yoshimi et al clearly show this by extensive immunophenotyping and molecular analyses. The authors go on to provide evidence that the CD34+ compartment contains the disease-initiating cells, demonstrating the potential of this model to hone in on this yet-to-be-defined population.
The preclinical testing of therapeutic agents in PDXs is becoming more common and may ultimately become a prerequisite for translation into the clinic. The authors demonstrate the feasibility of their CMML xenograft model for this purpose, testing the efficacy of the JAK2/FLT3 inhibitor, pacritinib. A number of features of this model make it particularly useful for preclinical studies, including its recapitulation of clinical, morphologic, and immunophenotypic disease features and the short disease latency, which allows for a rapid experimental readout. Additionally, evidence of aberrant DNA methylation has led to the testing of hypomethylating agents for the treatment of CMML and JMML,2,3 but the gradual impact of these therapies on disease burden generally precludes in vitro study of primary patient samples, which cannot be readily maintained in culture long term. Therefore, in vivo testing in xenografts represents the best option for preclinical testing of such epigenetically targeted therapies.
Although this model represents a major leap forward in the study of CMML and JMML, it also opens the door to new questions. Given the relatively small sample size, the authors were not able to correlate engraftment level or disease features to patient characteristics, prognosis, or mutation status. The efficiency of this model will make such future studies feasible and informative. Further, epigenetic factors are likely integral to the disease establishment, maintenance, and progression, yet how xenotransplantation impacts the tumor’s epigenetic landscape is unknown and could have an important impact on biological studies and preclinical testing of epigenetically targeted agents and, therefore, represents an important future application of this model.
Additionally, although the authors took advantage of the GM-CSF hypersensitivity characteristic of CMML and JMML2,3 by using the NSG-SGM3 strain that expresses human GM-SCF, the forced continual supraphysiologic cytokine levels could have some unintended effects. For example, sustained overexpression of human GM-CSF could blunt the efficacy of agents targeting the GM-CSF/STAT5 pathway, akin to the impedance of FLT3 inhibition efficacy due to increased expression of FLT3 ligand in acute myeloid leukemia patients.9 Newer strains of immunodeficient mice now exist that have key non–cross-reactive human cytokines knocked into the corresponding murine locus, such as the MITRG/MISTRG strains.10 Putting these cytokines under the control of endogenous promoters leads to more physiologic levels that could facilitate CMML/JMML engraftment without the aberrant overstimulation of the GM-CSF pathway resulting from constitutive transgenic overexpression.
In all, the development of the efficient and robust CMML and JMML xenograft model reported by Yoshimi et al is a significant advancement in the field, providing an ideal model for biological studies and preclinical testing of novel therapies for these clinically challenging neoplasms.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal