In this issue of Blood, Nieborowska-Skorska et al show that exposure of myeloproliferative neoplasm (MPN) cells to the JAK inhibitor, ruxolitinib, results in blockage of DNA damage repair pathways, thereby exacerbating double-strand DNA breaks and creating a synthetic lethal susceptibility to poly-ADP-ribose polymerase (PARP) inhibitors. Consequently, the combination of ruxolitinib with PARP inhibitors leads to elimination of quiescent and proliferating MPN leukemic stem/progenitor cells in vitro and in mouse models.1
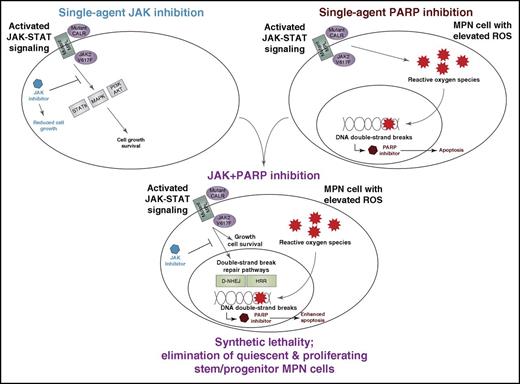
Synthetic lethality from combination of JAK and PARP inhibitors in MPN. Dysregulated JAK signaling from mutation of JAK2, MPL, or CALR leads to enhanced signaling through downstream pathways such as STATs, MAP kinase, and PI3K/AKT, which are well known to activate cell proliferation and survival programs. It has also recently been shown that this dysregulated JAK signaling leads to increased levels of ROS, which stall DNA replication forks and lead to increased levels of DNA double-strand breaks. These findings have been exploited to show that JAK inhibition lowers levels of DNA repair pathways, particularly BRCA-dependent HRR and D-NHEJ, which are important for DNA double-strand break repair and, in doing so, creates a synthetic lethal sensitivity to PARP inhibitors. This JAK/PARP inhibitor combination is sufficient to eliminate quiescent and proliferating MPN leukemic stem progenitor cells in vitro and in vivo.
Synthetic lethality from combination of JAK and PARP inhibitors in MPN. Dysregulated JAK signaling from mutation of JAK2, MPL, or CALR leads to enhanced signaling through downstream pathways such as STATs, MAP kinase, and PI3K/AKT, which are well known to activate cell proliferation and survival programs. It has also recently been shown that this dysregulated JAK signaling leads to increased levels of ROS, which stall DNA replication forks and lead to increased levels of DNA double-strand breaks. These findings have been exploited to show that JAK inhibition lowers levels of DNA repair pathways, particularly BRCA-dependent HRR and D-NHEJ, which are important for DNA double-strand break repair and, in doing so, creates a synthetic lethal sensitivity to PARP inhibitors. This JAK/PARP inhibitor combination is sufficient to eliminate quiescent and proliferating MPN leukemic stem progenitor cells in vitro and in vivo.
Single-agent therapies for cancer have largely provided only transient benefit, even when targeted to validated oncogenic drivers due to tumor heterogeneity, tumor cell-intrinsic feedback loops, and microenvironmental signals among other factors. Myeloproliferative neoplasms are a prime example of diseases with well-known oncogenic pathways in which single-agent targeting of those pathways has led to suboptimal responses. This experience indicates that drug combinations are needed, but testing of every possible drug pair is untenable. It is, therefore, critically important to identify the most promising drug combinations for advancement into clinical trials. A variety of strategies exist for prioritization of drug pairs, and this study by Nieborowska-Skorska and colleagues uses a strategy in which empirical observation of the impact of an established drug on biological responses within the target cell has led to identification of pathways that might be exploited in a synthetic lethal manner.
The classical MPNs (polycythemia vera, essential thrombocythemia, and primary myelofibrosis) are caused by exclusive mutations in JAK2, MPN, or CALR, which collectively result in JAK-STAT pathway activation in ∼80% to 90% of cases through distinct mechanisms induced by each mutation. Mutations can also be observed in epigenetic-regulating and splicesome genes, which may play important roles in disease initiation, disease progression, or both.2,3 Inhibitors of JAK kinases, such as ruxolitinib, momelotinib, and pacritinib, have resulted in some clinical benefit for patients; however, these agents have rarely led to durable or complete remissions for patients, highlighting the need for combination therapies that more durably and completely manage or eradicate disease.4
Many forms of cancer involve a delicate balance between increased levels of DNA damage, often induced by elevated levels of reactive oxygen species (ROS) found in tumor cells, and enhanced activity of DNA damage repair pathways to maintain sufficient genomic integrity to preserve intact tumor cell cycling.5 Leukemic cells from MPN patients have been shown to exhibit elevated levels of ROS,6 which lead to stalled DNA replication forks and increased frequency of DNA double-strand breaks (DSBs).7 The phenomenon of increased DSBs in cancer has been shown to lead to PARP inhibitor susceptibility in certain forms of cancer with mutations in DNA repair enzymes such as BReast CAncer susceptibility gene 1/2 (BRCA1/2), and this PARP inhibitor synthetic lethality has also been suggested as a potential strategy in other settings with dysregulation of DNA repair processes due to alternative mechanisms.8,9
In the present study, the prior observation of increased ROS and DNA double-strand breaks in MPN cells led the authors to investigate the sensitivity of MPN cells to PARP inhibitors as well as the impact of JAK inhibition on DNA repair pathway gene expression. They found modest PARP inhibitor sensitivity of unperturbed MPN cell lines, and MPN primary specimens showed variable baseline sensitivity. Interestingly DNA repair pathway genes, particularly genes involved in the DNA double-strand repair pathways of BRCA-dependent homologous recombination repair (HRR) and DNA-dependent protein kinase-mediated nonhomologous end-joining (D-NHEJ), were elevated in MPN cells expressing mutated JAK2, CALR, and MPL. Furthermore, treatment of MPN cells with ruxolitinib reduced expression levels of these HRR and D-NHEJ genes. Consequently, the treatment of MPN cells with the combination of ruxolitinib with a PARP inhibitor, either olaparib or BMN673, led to significantly increased levels of DNA double-strand breaks and apoptosis.
This led the study team to investigate the JAK/PARP inhibitor combination with and without hydroxyurea in patient sample colony assays, a syngeneic mouse model of retrovirally expressed and transplanted JAK2-V617F, and in an MPN patient xenograft mouse model. In all cases, enhanced drug activity was seen with JAK-plus-PARP inhibitor treatment with the addition of hydroxyurea, further enhancing antitumor effects. Importantly, by examining lineage negative cells that were CD34-positive/CD38-negative in human xenograft samples or Sca1-positive/Kit-positive in the murine syngeneic model, the data revealed that the combination of JAK with PARP inhibitors eliminated leukemia stem/progenitor cells in the in vivo mouse models. This was true for both proliferating and quiescent stem/progenitor cells in the human xenograft model, using dye dilution of cell trace violet to monitor the cell proliferative state (see figure).
In summary, this study proposes a promising new drug combination that has the potential to target the MPN leukemia stem/progenitor cell populations. The enhanced activity of this combination is based on a synthetic lethal vulnerability created by enhanced ROS and DSBs in MPN cells combined with the capacity of JAK inhibition to target both oncogenic cell survival pathways and the upregulated DNA repair processes that maintain MPN cell viability in the face of elevated ROS. The overall findings of this study point to significant drug synergy of this combination both in vitro and in vivo, offering ample preclinical evidence to support a path for clinical testing of the JAK and PARP drug combination strategy in myeloproliferative neoplasms.
Conflict-of-interest disclosure: J.W.T. receives research support from Agios Pharmaceuticals, Array Biopharma, Aptose Biosciences, AstraZeneca, Constellation Pharmaceuticals, Genentech, Gilead, Incyte Corporation, Janssen Pharmaceuticals, Seattle Genetics, Syros, and Takeda Pharmaceutical Company and is a consultant for Leap Oncology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal