In this issue of Blood, Nazir et al demonstrate that activated protein C (aPC) protects from ischemia-reperfusion injury by suppressing the activation of NLRP3 inflammasomes, revealing a novel target of the anti-inflammatory pathway triggered by biased agonism of proteinase-activated receptor 1 (PAR-1).1
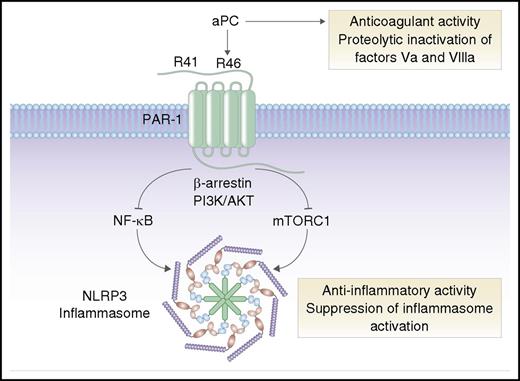
aPC is an anticoagulant serine protease that inactivates coagulation factors Va and VIIIa. In addition to its anticoagulant activity, aPC can induce anti-inflammatory cell signaling via cleavage of PAR-1 at arginine 46 (R46). Activation of PAR-1 by aPC triggers “biased” signaling via β-arrestin, phosphoinositide 3-kinase (PI3K), and AKT, leading to inhibition of NF-κB and mTORC1, and suppression of NLRP3 inflammasomes. In contrast, thrombin cleaves PAR-1 at arginine 41 (R41) and stimulates proinflammatory signaling pathways (not shown). Professional illustration by Somersault18:24.
aPC is an anticoagulant serine protease that inactivates coagulation factors Va and VIIIa. In addition to its anticoagulant activity, aPC can induce anti-inflammatory cell signaling via cleavage of PAR-1 at arginine 46 (R46). Activation of PAR-1 by aPC triggers “biased” signaling via β-arrestin, phosphoinositide 3-kinase (PI3K), and AKT, leading to inhibition of NF-κB and mTORC1, and suppression of NLRP3 inflammasomes. In contrast, thrombin cleaves PAR-1 at arginine 41 (R41) and stimulates proinflammatory signaling pathways (not shown). Professional illustration by Somersault18:24.
As a postdoctoral fellow in 1991, I remember being amazed by the discovery of PAR-1 by Shaun Coughlin and colleagues.2 Coughlin’s work was a scientific tour de force, identifying PAR-1 as the long-sought thrombin receptor and demonstrating a novel tethered ligand mechanism of proteolytic activation of a G-protein–coupled receptor. PAR-1 was the first identified member of the PAR family, which also includes PAR-2, PAR-3, and PAR-4. We know now that PARs can be activated or inactivated by a myriad of different serine proteases and matrix metalloproteinases, and that PARs can orchestrate differential “biased” cellular signaling responses to different proteases.3 For example, thrombin cleaves PAR-1 at arginine 41 and stimulates proinflammatory signaling pathways, whereas aPC cleaves at arginine 46 and directs anti-inflammatory responses4 (see figure).
Biased PAR-1 signaling by aPC or its analog 3K3A-aPC (which has reduced anticoagulant activity) protects from cerebral ischemia- reperfusion injury in animal models, and 3K3A-aPC is currently being evaluated in a phase 2 clinical trial in patients with ischemic stroke.4 aPC-mediated anti-inflammatory PAR-1 signaling also protects from myocardial reperfusion injury, but the mechanisms are incompletely understood. Nazir et al now demonstrate that aPC signaling via PAR-1 suppresses activation of NLRP3 inflammasomes in a murine model of myocardial reperfusion injury.
Inflammasomes are multimeric protein complexes that assemble in response to danger-associated molecular patterns, functioning as innate immune sensors that trigger caspase-mediated activation of interleukin-1β (IL-1β) and IL-18 and induce pyroptosis.5 Nazir et al found that treating mice with aPC decreased infarct size and inhibited expression of NLRP3, activation of caspase-1, and production of IL-1β and IL-18 after reperfusion injury in the heart or kidney. The protective effect of aPC in the heart was mimicked by 3K3A-aPC or parmodulin-2, a biased PAR-1 modulator, and lost in mice expressing a hyperactive NLRP3 variant. These findings are entirely consistent with prior work showing that NLRP3 inflammasome inhibition decreases infarct size and preserves cardiac function in animal models of myocardial infarction.6 Importantly, Nazir et al found inhibition of inflammasome activation by aPC was completely independent of its anticoagulant activity but required PAR-1-dependent signaling, suggesting that aPC-dependent biased signaling via PAR-1 restricts inflammasome assembly following reperfusion injury. Interestingly, aPC-mediated PAR-1 signaling in this setting was independent of the endothelial cell protein C receptor, a coreceptor required for biased PAR-1 activation by aPC in the brain and other tissues.4
The mechanism by which aPC suppresses inflammasome activation appears to involve inhibition of both NF-κB and the mechanistic target of rapamycin complex 1 (mTORC1) (see figure). Nazir et al found that deficiency of the endogenous mTORC1 inhibitor TSC1 blocked the ability of aPC to suppress NLRP3 inflammasomes in cultured cells, which suggests that biased aPC-PAR-1 agonism may limit mTORC1 signaling, possibly via effects on adenosine 5′-monophosphate–activated protein kinase.7 It remains to be determined whether the suppressive effect of aPC is limited to the priming step of inducing NLRP3 expression (which is known to be mediated by NF-κB) or also involves assembly of the inflammasome complex and/or inhibition of noncanonical inflammasome activation pathways.5
The new findings reported by Nazir et al expand our understanding of how an anticoagulant protease (aPC) and a facile, nuanced protease-sensitive G-protein–coupled receptor (PAR-1) can trigger potent anti-inflammatory and cytoprotective responses in the setting of reperfusion injury. The identification of the NLRP3 inflammasome as a key target of aPC-PAR-1 signaling suggests several potential therapeutic strategies, including aPC analogs, parmodulins, NLRP3 inhibitors, and perhaps even mTOR inhibitors, for the prevention of myocardial reperfusion injury. In this regard, it is encouraging that aPC was protective even when administered 30 minutes after the onset of reperfusion. Finally, these findings imply the potential to target biased PAR-1 signaling in other inflammatory conditions in which inflammasome activation is a prominent driver, such as tumor progression and metastasis, colitis, fibrosis, and aberrant wound healing.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal