To the editor:
Chimeric-antigen receptor T-cell (CART-cell) immunotherapy has proven clinical efficacy,1 particularly in B-cell leukemia and lymphoma.2-4 However, it causes a unique set of toxicities, the foremost of which is cytokine release syndrome (CRS).5,6 The pathophysiology of CRS remains poorly defined, but appears to result from the hyper-activation of CARTs strongly stimulated by the malignant cells, leading to massive production of several cytokines, including interleukin-6.2,3,6,7 This results in capillary leak and profound hypotension in the most severe cases.8,9 To understand the mechanisms of CART-induced CRS, we performed a comprehensive morphologic, immunophenotypic, and molecular analysis of tissues from a patient who succumbed to the syndrome. Detailed methods are described in the supplemental Appendix, available on the Blood Web site.
The patient was a 63-year-old man with CD19+/CD34+/CD10+/TdT+ B-cell acute lymphoblastic leukemia (B-ALL). Within 12 hours after infusion of CD19-directed CARTs (CTL019)1-3 he developed fever, mental status changes, multiorgan dysfunction, and hemodynamic instability. Cytokine serum concentrations, as assessed by multiplex bead assay, rose precipitously postinfusion with the overall profile (supplemental Figure 1A, supplemental Table 1) recapitulating CRS seen in CART-treated B-ALL patients.7 Accordingly, on day 3 postinfusion, his serum interleukin-6 (IL-6) concentration had markedly increased. However, the concentrations of the natural buffers of circulating IL-6, soluble IL-6 receptor and soluble glycoprotein 130,10 had decreased and increased very mildly, respectively. This discrepancy in cytokine vs cytokine receptor concentrations presumably led to higher amount of “unbuffered” IL-6 and possibly contributed to the patient’s demise. He was administered methylprednisone, the anti–IL-6 receptor antibody tocilizumab, and vasoactive agents, but succumbed to refractory hypotension on day 5.
Postmortem microscopic tissue evaluation (supplemental Figure 1B) revealed marked lymphoid cell infiltrates in the liver and lymph nodes. Immunohistochemistry demonstrated that the infiltrates contained almost exclusively enlarged CD3+CD7–-activated T cells (supplemental Figure 2). A subset stained for CD4 (supplemental Figure 3); the CD8 stain was suboptimal. Many lymphocytes expressed perforin, indicating activation of the cytotoxic program. Surprisingly, only rare CD79a+ (supplemental Figure 1B) CD10+ (supplemental Figure 3) B cells were seen in the liver, lymph nodes, and spleen, indicating a paucity of B-ALL blasts in these organs at the time of the patient’s death. In contrast, the bone marrow contained numerous CD10+/CD79a+ B-ALL blasts (supplemental Figure 1B).
Reverse-transcriptase quantitative polymerase chain reaction (qPCR)-based CD19 gene-expression analysis and clone tracking using IGH gene-focused next-generation DNA sequencing (NGS) confirmed the predominance of B-ALL cells in the bone marrow and their paucity in the solid organs. RT-qPCR detected a high copy number of CD19 messenger RNA in the bone marrow, but none in other tissues (supplemental Table 2). The more sensitive IGH-specific NGS assay easily detected the patient’s leukemic clone in peripheral blood drawn before CART infusion and all tissues (Figure 1A-B). The bone marrow contained ∼50 times more leukemic cells than the preinfusion peripheral blood (Figure 1C). The leukemic clone was also present in the liver and lymph nodes, albeit at much lower concentrations.
Organ distribution of leukemic clone and reactive CARTs. (A) IGH sequencing demonstrates dominance of the B-ALL clone (marked with a red dot) over all other B-cell clones (blue dots) in PB at autopsy (PB day 5 [d5]), bone marrow (BM), and several other tissues, including the lymph nodes (LN). The frequency of unique clonotypes as a proportion of all immunoglobulin H reads is plotted for the baseline blood sample (all x-axes) vs the day 5 autopsy tissues (y-axis). (B) The leukemic clone represents the largest fraction of B-cell clones in all tissues examined. (C) Quantitative analysis of the IGH sequencing data identifies the B-ALL clone predominantly in the BM and PB. Much lower concentrations of the leukemic clone were seen in the remaining examined tissues, including the LN and liver. (D) The T-cell clones that dominate the repertoires within the LN, liver, and BM (green triangles, blue circles, and red squares, respectively) originate predominantly from the CD8+ fraction of the infused CARTs, rather than the CD8– (E) fraction. (F) The most dominant clones and their frequencies in the LN, BM, and liver. The sharing among these organs of dominant clones indicates the presence of the same immune reaction, almost certainly being carried out by CARTs stimulated by CD19 expressed by B-ALL cells. (G) ISH identifies frequent CAR cells among the T-cell infiltrate in the LN and liver. MC, mononuclear cell; PB, peripheral blood.
Organ distribution of leukemic clone and reactive CARTs. (A) IGH sequencing demonstrates dominance of the B-ALL clone (marked with a red dot) over all other B-cell clones (blue dots) in PB at autopsy (PB day 5 [d5]), bone marrow (BM), and several other tissues, including the lymph nodes (LN). The frequency of unique clonotypes as a proportion of all immunoglobulin H reads is plotted for the baseline blood sample (all x-axes) vs the day 5 autopsy tissues (y-axis). (B) The leukemic clone represents the largest fraction of B-cell clones in all tissues examined. (C) Quantitative analysis of the IGH sequencing data identifies the B-ALL clone predominantly in the BM and PB. Much lower concentrations of the leukemic clone were seen in the remaining examined tissues, including the LN and liver. (D) The T-cell clones that dominate the repertoires within the LN, liver, and BM (green triangles, blue circles, and red squares, respectively) originate predominantly from the CD8+ fraction of the infused CARTs, rather than the CD8– (E) fraction. (F) The most dominant clones and their frequencies in the LN, BM, and liver. The sharing among these organs of dominant clones indicates the presence of the same immune reaction, almost certainly being carried out by CARTs stimulated by CD19 expressed by B-ALL cells. (G) ISH identifies frequent CAR cells among the T-cell infiltrate in the LN and liver. MC, mononuclear cell; PB, peripheral blood.
To evaluate the frequency of CARTs, the quantity of integrated chimeric-antigen receptor (CAR) was assessed by qPCR (supplemental Table 3) of isolated whole blood and tissue genomic DNA. The liver, lymph nodes, and post-CART infusion peripheral blood were highly enriched in αCD19-BB-ζ–marked CARTs. In contrast, the bone marrow contained little CAR signal. To characterize the clonal T-cell makeup in these tissues, we performed NGS of the T-cell receptor (TRB) gene repertoire in the liver, bone marrow, lymph nodes, and CD8+ and CD8– fractions of the infused CARTs (Figures 1D-E). This demonstrated a diverse TRB clonal repertoire among the infused CARTs, whereas the tissue samples contained somewhat more restricted repertoires. Of note, these tissues contained the same relatively dominant clones (Figure 1F), many of which were also identified in the infused T cells. Although some were detected in the bone marrow, this was at a much lower frequency. To determine if activation of native, CAR-untransduced T cells occurred as well, we performed in situ hybridization (ISH) using a CAR-specific RNA probe (Figure 1G). Although this analysis identified frequent CARTs within the lymph nodes and liver, many of the enlarged lymphocytes lacked CAR signal, indicating that CART activation is associated with coactivation of non-CARTs.
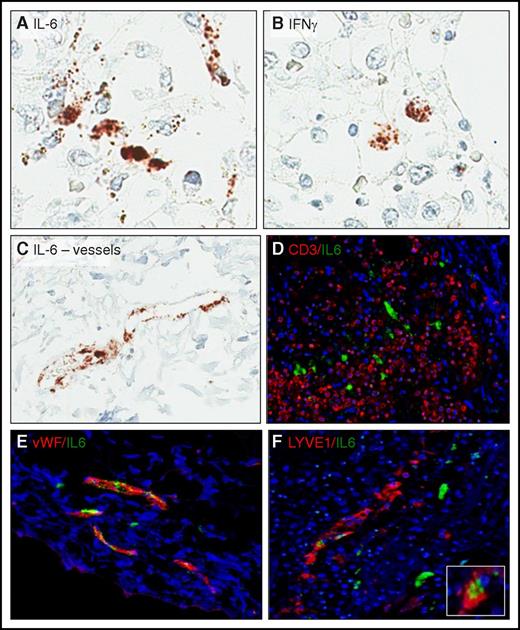
To further elucidate the molecular pathophysiology of CRS, we examined by RNA-ISH the expression of IL-6 (Figure 2), the key cytokine in this disorder.3,7,11 Although a subset of interstitial cells expressed IL-6, they were nonlymphoid, likely accessory, cells. Notably, vascular lining cells also expressed IL-6. Dual ISH hybridization corroborated the morphological impression, revealing a lack of costaining of IL-6 with CD3, confirming the non–T-cell nature of the IL-6–expressing interstitial cells. Dual ISH hybridization also confirmed endothelial cells as the IL-6 producers and indicated that the von Willebrand factor–positive blood vessel lining cells expressed the cytokine more robustly than LYVE1-positive lymphatic lining cells.
Cell-type source of CRS-associated cytokine in the lymph nodes. ISH was used to identify cells expressing IL-6 (A,C) and interferon-γ (B). (D) Lack of IL-6 (green) and CD3 (red) colocalization indicates that T cells/CARTs are not the source of IL-6. (E) Colocalization of IL-6 (green) with von Willebrand factor (vWF; red) expressed by blood vessel endothelium and (F) lymphatic vessel endothelial antigen 1 (LYVE1; red) confirms that endothelial cells, in particular blood vessel endothelial cells, are the key source of IL-6 in CRS. Inset: high-power view of the double-positive endothelial cells.
Cell-type source of CRS-associated cytokine in the lymph nodes. ISH was used to identify cells expressing IL-6 (A,C) and interferon-γ (B). (D) Lack of IL-6 (green) and CD3 (red) colocalization indicates that T cells/CARTs are not the source of IL-6. (E) Colocalization of IL-6 (green) with von Willebrand factor (vWF; red) expressed by blood vessel endothelium and (F) lymphatic vessel endothelial antigen 1 (LYVE1; red) confirms that endothelial cells, in particular blood vessel endothelial cells, are the key source of IL-6 in CRS. Inset: high-power view of the double-positive endothelial cells.
We next examined if immunosuppressive mechanisms became activated in an attempt to ameliorate the CRS-associated exuberant immune response. Metabolic degradation of tryptophan by indoleamine-2,3-dioxygenase 1 (IDO1) is one mechanism of inhibiting T-cell responses.12 ISH demonstrated high expression of IDO1 in the lymph nodes (supplemental Figure 4), suggesting that brisk tryptophan degradation was, indeed, involved in an attempt to control the patient’s CRS.
In this letter, we report the first tissue-based insights into the pathogenesis of CART -associated CRS. Our comprehensive analysis sheds light on the tissue distribution and clonal makeup of CARTs and the mechanisms of CRS. High tumor burden, although possibly insufficient per se to trigger CRS, appears nevertheless to be a prerequisite for severe CRS.7,13 We also demonstrated that CARTs administered to mice with a high,14 but not low,15 lymphoma tumor burden developed lethal CRS. Indeed, this patient had significant leukemic burden in the bone marrow at the time of death; the leukemic clone was also detected in peripheral blood. The marked CART infiltrates and molecular detection of the B-ALL clone suggest substantial leukemic involvement of the liver, lymph nodes, and spleen as well prior to the infusion. With the caveat of having only a snapshot of the dynamics at play, we found a remarkable discordance between the tissue localization of the CARTs and leukemic cells. Although the bone marrow was replaced by sheets of leukemic cells, CARTs were primarily localized to other organs, including the lymph nodes and liver. This disconnect may be explained by more effective activation of CARTs by B-ALL cells in lymph nodes and hepatic portal tracts, tissues that are more adapted to mounting protective immune responses than bone marrow, which is typically not a site of immune reactions. However, differences in CART19 trafficking could also have played a role. The observation of high concentrations of CARTs in bone marrow detected after 3 weeks,3 the earliest postinfusion time point to be evaluated, could be the result of either of these scenarios.
The combined tissue analysis with diverse methods clearly indicates that not only CARTs but also T cells without CAR became activated. The mechanisms of activation of these non-CARTs may result from “epitope spreading,”16,17 in which the previously silent native T cells became activated due to the stimuli generated by the activated CARTs, contributing to CRS on one hand, and likely to the anti-tumor response on the other.
Our data demonstrate that vascular endothelial cells and other non–T cells are the key source of IL-6. This observation radically expands our understanding of CRS, which has until now been thought to be largely a result of the interactions between malignant cells and CARTs. The direct implication of endothelial cells provides a feasible explanation for the marked hypotension seen in severe CRS and could explain why therapeutic targeting of IL-6 alone is frequently effective in ameliorating CRS symptoms. Our finding of increased IDO1 expression strongly suggests that homeostatic mechanisms have been activated in response to CRS-related hyperactivation of immune cells. Increased expression of IDO1 may be driven by increases in the proinflammatory cytokines IL-6 and interferon-γ.18 Therefore, future CRS therapies may include efforts to boost these immune response–limiting mechanisms.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank F. Chen and N. Kengle for serum cytokine detection studies, I. Kulikovskaya and M. Gupta for qPCR, V. Gonzalez for data management, and J. Finklestein for sample management.
This work was supported in part by the Penn-Novartis Alliance and Berman Family Funds.
Contribution: A.E.O., K.M., S.F.L., J.J.M., and M.A.W. performed the experiments, analyzed the data, and contributed to writing the manuscript; N.V.F. and D.L.P. took care of the patient, provided clinical information, and contributed to writing the manuscript; C.H.J. contributed to writing the manuscript; A.E.O. and M.A.W. wrote the manuscript; and M.A.W. directed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariusz A. Wasik, Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center, 280 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: wasik@mail.med.upenn.edu.

![Figure 1. Organ distribution of leukemic clone and reactive CARTs. (A) IGH sequencing demonstrates dominance of the B-ALL clone (marked with a red dot) over all other B-cell clones (blue dots) in PB at autopsy (PB day 5 [d5]), bone marrow (BM), and several other tissues, including the lymph nodes (LN). The frequency of unique clonotypes as a proportion of all immunoglobulin H reads is plotted for the baseline blood sample (all x-axes) vs the day 5 autopsy tissues (y-axis). (B) The leukemic clone represents the largest fraction of B-cell clones in all tissues examined. (C) Quantitative analysis of the IGH sequencing data identifies the B-ALL clone predominantly in the BM and PB. Much lower concentrations of the leukemic clone were seen in the remaining examined tissues, including the LN and liver. (D) The T-cell clones that dominate the repertoires within the LN, liver, and BM (green triangles, blue circles, and red squares, respectively) originate predominantly from the CD8+ fraction of the infused CARTs, rather than the CD8– (E) fraction. (F) The most dominant clones and their frequencies in the LN, BM, and liver. The sharing among these organs of dominant clones indicates the presence of the same immune reaction, almost certainly being carried out by CARTs stimulated by CD19 expressed by B-ALL cells. (G) ISH identifies frequent CAR cells among the T-cell infiltrate in the LN and liver. MC, mononuclear cell; PB, peripheral blood.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/23/10.1182_blood-2017-08-802413/4/m_blood802413f1.jpeg?Expires=1765906693&Signature=D2kofcCHa68cD7wrSdvrtXXS2bXNLLzBNhx52kgmZAP66-jNrlvLqRKmc7iQ21TNQHZ6u2W-sHxLSmDDhkkf7hG26EpN8TZKItVr6BP-hsAM1Gy87O2UdzM6Buvg8BrygXY-6ZLV66n57n8yr8vKmwja03Kg6dB3FwRZag21wvqphvnjio5TyOGJSelaMW9zBUdwuYSYO~v5kBYSUiJE5aa0T7HTsPmOP9qDHuSkg9e01RnJGdflxxgb3w57BwcgNCZeB9PYSEkguk3mfw74BL~zk6iB4Sb4T4QdvTjKCLG85Pq-Jpo-t1T8quWVkRJ~AygoM7XTtMp5IBDtj3xeYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal