In this issue of Blood, Haining et al reveal an unexpected role for platelet C-type lectin-like receptor-2 (CLEC-2) in thrombosis, which is independent of its hemi-immunoreceptor tyrosine-based activation motif (hemITAM) signaling.1
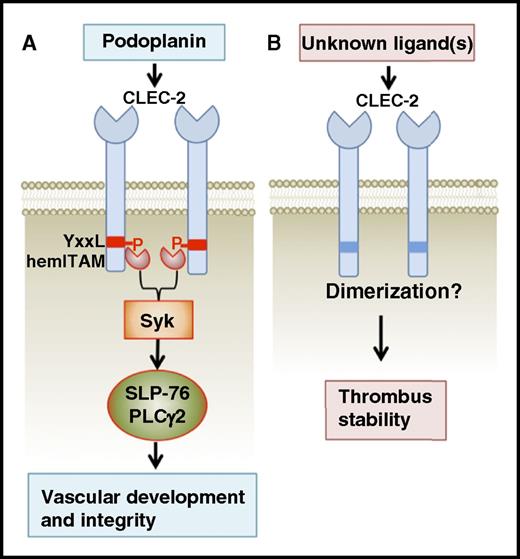
Model showing a hemITAM-dependent function of platelet CLEC-2 in vascular development and integrity (A), as well as a hemITAM-independent role of platelet CLEC-2 in thrombus formation (B). P, phosphorylated.
Model showing a hemITAM-dependent function of platelet CLEC-2 in vascular development and integrity (A), as well as a hemITAM-independent role of platelet CLEC-2 in thrombus formation (B). P, phosphorylated.
CLEC-2 is a highly expressed platelet receptor.1,2 Its intracellular domain contains a unique single YxxL motif (hemITAM) that is capable of activating platelets through downstream signaling effectors Syk and SLP-76 upon ligand engagement of a CLEC-2 dimer. Platelet CLEC-2 is involved in 2 important biological processes: blood/lymphatic vessel separation and thrombosis. The former function has been well characterized in mice lacking platelet CLEC-2 or its intracellular signaling molecules Syk or SLP-76 because these mutant mouse models share a distinct blood-lymphatic mixing vascular phenotype. This indicates a critical function of hemITAM signaling for CLEC-2: to regulate blood-lymphatic separation during lymphatic vascular development.3 The blood-lymphatic mixing phenotype is also seen in mice lacking the O-glycoprotein podoplanin,4 the only known physiological ligand of CLEC-2. Podoplanin is specifically expressed on lymphatic endothelial cells. Therefore, these data indicate that platelet activation mediated by CLEC-2 hemITAM signaling upon binding to lymphatic endothelial podoplanin is essential for forming an independent lymphatic vascular system. Other than this well-defined role in vascular development, mice with CLEC-2–deficient platelets also exhibit impaired platelet aggregate formation and lower susceptibility to arterial thrombus formation relative to wild-type controls.5,6 Podoplanin does not seem to be involved in platelet CLEC-2–mediated thrombus formation as it is expressed neither on blood endothelial cells nor on other blood cells under physiological conditions.7 But, whether hemITAM-mediated signaling is required in this biological process remains to be determined.
In this study, Haining et al generated a novel knock-in mouse model that expresses a mutant form of CLEC-2 with its cytoplasmic hemITAM YxxL motif mutated (Y7A KI). The Y7A KI platelets express normal surface levels of CLEC-2 as well as other platelet surface receptors. Y7A KI platelets display normal responses to classic agonists such as thrombin and collagen, but have a defective response to the CLEC-2 agonist rhodocytin. By binding to CLEC-2, both rhodocytin and podoplanin activate platelets through hemITAM-dependent signaling. Like CLEC-2 knockout (KO) mice, Y7A KI mice have the blood-lymphatic mixing phenotype, confirming the critical role of the hemITAM-dependent signaling of CLEC-2 in promoting separation of lymphatic vessels from the blood vessel during development. However, unlike CLEC-2 KO mice, Y7A KI mice have normal bleeding time and thrombus formation, in 2 different arterial thrombosis models, similar to that of wild-type controls. Importantly, treatment of Y7A KI mice with the Fab′ fragments of the anti-CLEC-2 antibody, INU1, which does not cross-link CLEC-2 to induce hemITAM signaling, reduces thrombus formation, revealing a hemITAM signaling–independent role for CLEC-2 in thrombosis.
Haining et al show that CLEC-2 is required for platelets to interact with other platelets adherent to sites of vascular injury within the vessel to form thrombus, which again raises the often-asked question: how does platelet CLEC-2 contribute to thrombus formation (see figure)? Podoplanin is not known to be present on platelets or other blood cells under normal conditions.7 In addition, the INU1 Fab′ fragments block aggregate formation of Y7A KI platelets on collagen under flow conditions in vitro. These data are suggestive of additional endogenous ligand(s) for platelet CLEC-2. There have been several attempts to identify new ligands/counterreceptors for platelet CLEC-2, but none of the studies appear to be conclusive. Thus, future studies are required to reveal the mysterious blood-borne CLEC-2–interacting molecule(s) and its binding site on CLEC-2. Podoplanin and CLEC-2–mediated platelet activation is also critical for protecting vascular integrity in many important organs, such as lymph nodes and the brain.7,8 This hemITAM signaling–independent platelet CLEC-2 function is neither required for vascular development/integrity nor involved in hemostasis, that is, initial platelet adhesion. Therefore, it represents an attractive therapeutic target for development of an effective and safe antithrombosis therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal