A number of disease- or host-related markers have been proposed to predict treatment outcome in relapsed/refractory Hodgkin lymphoma (HL). In this issue of Blood, Moskowitz et al demonstrate that fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) performed in sequence during second-line treatment recapitulates all of them.1
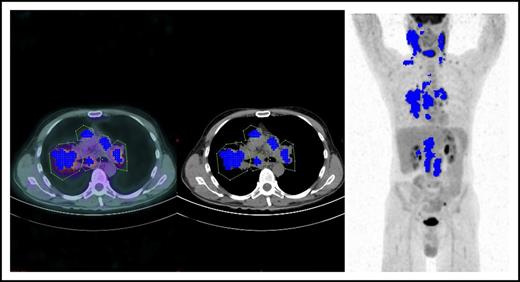
Tumor segmentation in an HL patient showing 5 sizeable mediastinal lymph nodes (courtesy of Salim Kanoun).
Tumor segmentation in an HL patient showing 5 sizeable mediastinal lymph nodes (courtesy of Salim Kanoun).
In the era of new drugs, high-dose chemotherapy (HDC) followed by autologous stem cell transplantation (ASCT) remains the best treatment option for relapsed/refractory HL. Intensity and duration of second-line salvage therapy do not affect treatment outcome if HDC plus ASCT follows chemotherapy.2 Quite recently, an international consortium for prognostic factors modeling in relapsed/refractory HL (the RisPACT consortium) retrospectively evaluated the impact of 23 risk factors (RF) on ASCT outcome in 546 patients with relapsed/refractory HL in 9 prospective trials.3 Stage IV, time to relapse ≤3 months, Eastern Cooperative Oncology Group performance status ≥1, a bulk nodal lesion >5 cm, and an inadequate therapy response, assessed with a CT or PET/CT before ASCT, proved to be, in multivariate analysis, the only RF predictive of progression-free survival. Different groups have demonstrated that interim PET (iPET) before ASCT in relapsed/refractory HL had a predictive value (PV) independent from other RFs,4 similar to that during doxorubicin, bleomycin, vinblastine, dacarbazine treatment, where iPET proved the prognostic marker with the highest PV, irrespective of other RFs included in the International Prognostic Score.5 The physiopathological mechanism underpinning this very high PV in HL is complex and incompletely understood. The great avidity for FDG reported in HL has been allegedly attributed to the high glycolytic activity of the microenvironment (ME) cells. In the latter, as in neoplastic cells, the glycolysis proved as high as 200 times that of normal tissue. ME is, by far, the largest cellular fraction in HL tissue, and its metabolic activity is sustained by a cytokine network produced by few neoplastic Hodgkin and Reed-Sternberg (HRS) cells. On the other hand, chemotherapy-induced HRS cell kill switches off the FDG uptake by ME cells. Thus, the latter work in HL as a PET signal amplifier. Quite recently, however, other explanations have been proposed: the CD8+ tumor-infiltrating lymphocytes and the reactive lymphocytes expressing the surface antigen programmed death-1 (PD-1), implicated in naive host immunity against the tumor, have also been reported to demonstrate great FDG avidity.6 Because high PD-1 expression by ME cells of HL was shown to predict a poor treatment outcome,7 the intensity of the PET signal could also depend on the reactivity (and effectiveness) of the host immune response against the tumor.
In the current issue, Moskowitz et al demonstrate that metabolic tumor volume (MTV) in relapsed/refractory HL, measured before treatment onset in baseline FDG-PET (bMTV), proved the most powerful predictor of final treatment outcome. Very interestingly, bMTV and iPET behaved as independent prognostic factors, and the former improved the PV of the latter. This, however, is not completely unexpected, because the combination of baseline and interim PET seems to recapitulate the RF of the RisPACT study: bMTV in baseline PET portrays tumor burden (bulk and stage IV disease), whereas iPET is able to detect low chemosensitivity (inadequate response to treatment/chemotherapy resistance and possibly an inadequate immune response by the host).
MTV is one of the most promising applications of quantitative FDG-PET scan (QPET) reading.8 FDG-PET is indeed an intrinsically quantitative imaging tool capable, in theory, of detecting and measuring all the voxels contained within the tumor, thus portraying, in a single shot, the overall functionally active tumor burden. The QPET readout consists of a computer-assisted mathematical calculation of numerical variables, such as MTV and total lesion glycolysis, moving from a unit of measure, the standardized uptake value (SUV), which is the ratio between the measured activity and the injected activity per voxel, normalized to body surface area. After manual contouring of every single tumor mass, the algorithm to compute MTV starts with tumor segmentation, that is, the decomposition in a 3-dimensional space of every Voxel measured within a contoured tumor mass showing an SUV value between the SUV maximum (SUVmax) and the SUV threshold (see figure). The latter could be a fixed percentage of SUVmax, for instance, 41% as in the Moskowitz et al article, or an absolute value. Thus, QPET could achieve 2 ambitious goals at the same time: (1) providing a partially operator-independent PET scan reading, and (2) transforming a descriptive imaging report into a number, as in other prognostic biochemical markers. However, several hurdles still preclude the use of bMTV as a reliable and reproducible biomarker in clinical practice, as recently pointed out by the same authors of the article.9 As a matter of fact, a number of biological and physical factors as well as technical errors could undermine the absolute SUV measurement, with variability ranging between 15% and 75% when this value is obtained from different PET scanners with different hardware in multicenter trials.10 Nonetheless, several newer experiences have been published, demonstrating that this variability could be decreased if the procedure for PET scanning is harmonized among sites and if PET/CT scanners are intercalibrated. Moreover, a debate still exists on the threshold to be used in SUVmax measurement and on the best segmentation algorithm. Because it is not possible to evaluate, from a methodological point of view, the accuracy in tumor delineation of the single segmentation algorithm, a plethora of them have been used to determine MTV with different results. Finally, MTV behaves as a continuous nonlinear variable, and therefore a number of different cut-off values to predict treatment outcome have been proposed so far, making it useless for clinical purposes. As a matter of fact, the bMTV prognostic role has so far only been demonstrated in retrospective studies or single-center studies, in which the abovementioned methodological flaws are bypassed or minimized. Therefore, prospective studies with calibrated scanners with homogeneous hardware equipment adopting the same software for MTV computing and the same SUV threshold are urgently needed.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal