In this issue of Blood, Niss et al provide evidence of significant myocardial fibrosis and link it to diastolic dysfunction, a known predictor of mortality in sickle cell disease (SCD).1
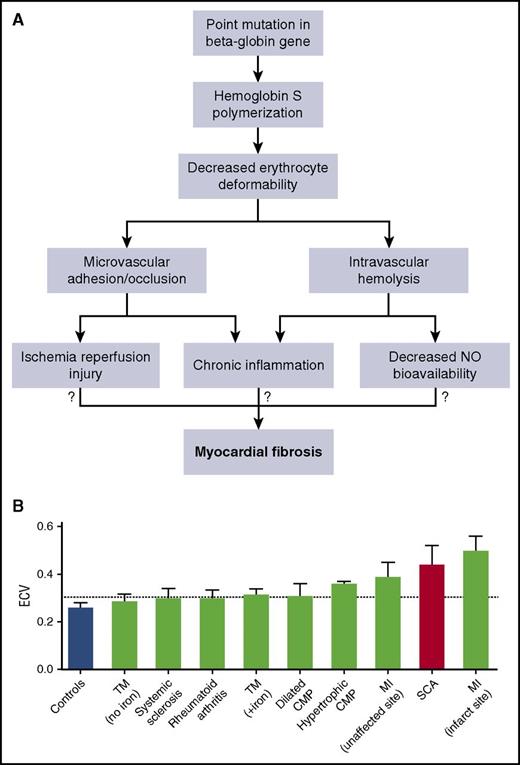
Myocardial fibrosis: a concerning finding without an etiology. (A) Despite the “simple” point mutation and hemoglobin S formation that underlies SCD, there are numerous adverse cellular and biochemical changes. Hemoglobin S polymerization leads to decreased erythrocyte deformability and eventually microvascular occlusion and intravascular hemolysis. Both microcirculatory occlusion and intravascular hemolysis act in concert to cause tissue ischemia-reperfusion injury, chronic inflammation, and decreased NO bioavailability. We do not know which abnormality, if any, leads to myocardial fibrosis. (B) ECV quantification in SCD is exceeded only by ECV quantification at the site of infarcted myocardium. CMP, cardiomyopathy; SCA, sickle cell anemia; TM, thalassemia major (+iron, indicates presence of myocardial iron overload). See Figure 2C in the article by Niss et al that begins on page 205.
Myocardial fibrosis: a concerning finding without an etiology. (A) Despite the “simple” point mutation and hemoglobin S formation that underlies SCD, there are numerous adverse cellular and biochemical changes. Hemoglobin S polymerization leads to decreased erythrocyte deformability and eventually microvascular occlusion and intravascular hemolysis. Both microcirculatory occlusion and intravascular hemolysis act in concert to cause tissue ischemia-reperfusion injury, chronic inflammation, and decreased NO bioavailability. We do not know which abnormality, if any, leads to myocardial fibrosis. (B) ECV quantification in SCD is exceeded only by ECV quantification at the site of infarcted myocardium. CMP, cardiomyopathy; SCA, sickle cell anemia; TM, thalassemia major (+iron, indicates presence of myocardial iron overload). See Figure 2C in the article by Niss et al that begins on page 205.
SCD is a model for chronic and diffuse vasculopathy resulting in tissue infarction and ischemia-reperfusion injury.2,3 Much attention has been placed on diffuse endothelial disease in SCD. More recently, diastolic heart failure has been recognized as a significant cause of morbidity and mortality in the general population and more specifically in SCD patients.4 However, there has been a paucity of work aimed at understanding the mechanism underlying cardiac enlargement and diastolic dysfunction, and how they are related to mortality in this disease population. Recent evidence from this same group, using the Berk-SS mouse model, suggests myocardial fibrosis results in a restrictive cardiomyopathic process.5 Niss and colleagues use cardiac T1 mapping, a noninvasive measure of myocardial fibrosis,6 to demonstrate that myocardial fibrosis is associated with diastolic dysfunction, an important first step and consistent with their prior animal studies. This also gives researchers and clinicians the ability to assess and longitudinally track myocardial fibrosis, test mechanistic hypotheses about myocardial fibrosis, and develop treatments aimed at slowing or reversing its development.
Diastolic heart function has both active and passive components. The myosin-actin interaction during relaxation is an active process and myocyte-fibrous tissue interaction accounts for the passive relaxation process. Cardiac T1 mapping is a technique by which measurements of extracellular volume fraction (ECV), using gadolinium-based magnetic resonance contrast agents, have been linked to myocardial fibrosis. Measurements of cardiac ECV are used for disease tracking and prognosis in hypertrophic cardiomyopathy, sarcoidosis, and, more recently, heart failure with a preserved ejection fraction, a model of heart failure that has been ascribed to SCD.7 Similar to these other myopathic processes, the development of myocardial fibrosis in this patient population is likely chronic and thus important to recognize because improved survival is unmasking chronic disease that burdens patients as they age. This study found an effect of age on the relationship between ECV and tricuspid regurgitant jet velocity. Are the development of pulmonary hypertension and diastolic dysfunction concordant and, if so, what drives this process?
SCD stems from a single point mutation that leads to the formation of hemoglobin S, which polymerizes upon deoxygenation and leads to decreased erythrocyte deformability. How does this fundamental change in the erythrocyte lead to the development of myocardial fibrosis? Decreased erythrocyte deformability and increased erythrocyte-endothelial adhesion create the substrate for microvascular occlusion and tissue ischemia-reperfusion injury. Chronic hemolysis releases free hemoglobin and arginase, both of which act to decrease nitric oxide (NO) bioavailability and increase redox stress. Intravascular hemolysis and microvascular occlusion also lead to innate immune system activation and chronic inflammation. All of these biochemical, molecular, and cellular changes funnel into end-organ ultrastructural changes and end-organ dysfunction. Which, if any, of these abnormalities leads to myocardial fibrosis and restrictive cardiomyopathy in SCD (see figure panel A)?
Work in the preclinical Berk-SS mouse model, by this same group, found myocardial fibrosis was increased, and not solely due to anemia and hypoxia. Anemia acts in combination with cardiac chamber enlargement, microvascular occlusion, ischemia, and oxidative stress, leading to myocardial fibrosis. Furthermore, there are signature changes in the myocardial transcriptome consistent with abnormal regulation of oxidative stress, extracellular matrix formation, angiogenesis, lipid metabolism, and neurotransmission. SCN4A, SCN4B, and KCNJ2, which are genes associated with long-QT syndrome and sudden death, were significantly downregulated. Ion-channel dysregulation is consistent with their mouse clinical data, demonstrating prolongation of the corrected QT interval and fatal arrhythmias, an important link between myocardial fibrosis and channelopathies directly related to sudden cardiac death.
Sudden death is a significant problem in adult patients with SCD, but the etiology is unknown and likely multifactorial.2 Chronic fibrotic change in both the conduction system and smaller coronary arteries was found in an autopsy specimen from a patient with SCD who died suddenly.8 Could ECV and diastolic dysfunction found in this study be related to fibrotic changes found in this autopsy specimen? Niss and colleagues found that ECV and diastolic dysfunction were both associated with severity of anemia and cardiac chamber size, which is consistent with the Berk-SS mouse model. However, neither the “number of painful events” nor acute chest episodes were associated with ECV or diastolic dysfunction, both of which are thought to be associated with microvascular occlusion. Although this raises more questions about etiology and pathophysiology, this is an important first step toward characterizing and better understanding diastolic dysfunction and the reason it is an independent risk factor for mortality. Figure 2 of the article by Niss et al (see figure panel B) is an important and sobering graphic. The ECV quantification in SCD patients is surpassed only by measurements at the site of myocardial infarction (MI) in patients who have suffered acute MI.
There are important clinical aspects related to T1 mapping in this population. Myocardial iron deposition affects T1 relaxation measurements by lowering native T1 relaxation time, which may lead to an underestimation of myocardial fibrosis. Myocardial iron deposition is rare in SCD, even in chronically transfused patients.9 Use of gadolinium-based contrast agents has generally been avoided in SCD patients due to preexisting renal dysfunction in SCD; however, with adequate prescreening of renal function, complications can be avoided. The theoretical concern for increased pain crisis frequency after gadolinium administration has not been supported in clinical studies but should be tracked and reported.1 Therefore, ECV quantification using gadolinium-based T1 mapping can be used as a biomarker of chronic myocardial disease, followed longitudinally, and is noninvasive and radiation free.
As survival has improved in patients with SCD and we transition pediatric patients to adult hematology centers, chronic complications of hemolysis, inflammation, microvascular occlusion, ischemia, and reperfusion are coming to bear. Treatment for cardiac fibrosis and diastolic function remain challenging. Cardiac T1 mapping would allow us to monitor progression of fibrosis and its response to current therapies such as hydroxyurea, transfusion, and more recent therapeutic agents modulating erythrocyte adhesion and oxygen dissociation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal