In this issue of Blood, Harrison et al report the results of the first randomized trial comparing ruxolitinib and best available therapy (BAT) in patients with essential thrombocythemia (ET) resistant or intolerant to hydroxycarbamide (HC). The study suggests that ruxolitinib is not superior to current second-line treatments for ET.1
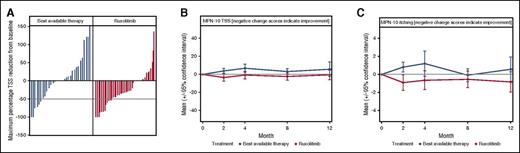
Changes in ET-related symptom burden during year 1 of the MAJIC-ET trial. (A) A waterfall plot of maximum percentage change in the MPN-10 TSS score; dotted line indicates 50% reduction in TSS. (B) The mean MPN-10 TSS throughout the first year of the trial; there was a consistent trend for reduction for ruxolitinib. (C) The mean MPN-10 score for itching during the first 12 months of the MAJIC-ET trial. See Figure 3 in the article by Harrison et al beginning on page 1889.
Changes in ET-related symptom burden during year 1 of the MAJIC-ET trial. (A) A waterfall plot of maximum percentage change in the MPN-10 TSS score; dotted line indicates 50% reduction in TSS. (B) The mean MPN-10 TSS throughout the first year of the trial; there was a consistent trend for reduction for ruxolitinib. (C) The mean MPN-10 score for itching during the first 12 months of the MAJIC-ET trial. See Figure 3 in the article by Harrison et al beginning on page 1889.
Ruxolitinib, the first-in-class JAK1/2 inhibitor, was demonstrated to be effective in reducing splenomegaly and constitutional symptoms in patients with primary myelofibrosis,2,3 and in those with polycythemia vera (PV) resistant or intolerant to HC4,5 in randomized phase III clinical trials. On this basis, the drug was approved for clinical use in these diseases both in the United States and in Europe. ET differs from the other chronic myeloproliferative neoplasms (MPNs) in that it has a more indolent clinical course, less severe splenomegaly and clinical symptoms with a life expectancy similar to that of normal individuals. On the other hand, ET patients show an increased risk of thrombosis and bleeding, and a tendency to progress to post-ET myelofibrosis (MF) with rare progression to acute myeloblastic leukemia (AML).6 Ruxolitinib was never evaluated in randomized trials in ET, and this study (MAJIC-ET trial) addresses this gap. The study analyzed several important clinical end points for ET patients, including thrombosis, hemorrhage, clinical symptoms, and disease progression. The primary outcome measure was achievement of complete response (CR), as defined by normal platelet and white cell count and normal spleen size. At 1 year, CR was achieved in 46% of patients in the ruxolitinib arm in comparison with 44% in the BAT arm, without a significant difference between the 2 groups. No difference was observed in the rates of thrombosis, hemorrhage, and disease transformation after 2 years of follow-up. However, some disease-related symptoms, measured by standardized questionnaires, improved in patients receiving ruxolitinib rather than BAT. Total symptom score (TTS) reduction at any point during the first year of treatment was significantly greater for ruxolitinib than it was for BAT (see figure, panel A) and longitudinally, mean TSS and the individual symptom of pruritus were significantly lower for ruxolitinib than for BAT (see figure, panels B-C). As was expected, ruxolitinib treatment was more frequently associated with anemia and infections than was BAT, even though rates of discontinuation or treatment switching did not differ between the 2 trial arms. Thus, there were more shadows than lights in this study, supporting the conclusion that ruxolitinib did not improve treatment efficacy compared with BAT for most clinically relevant events. However, confirmatory studies would be welcome, especially considering the limitations of the MAJIC-ET trial. Albeit randomized, this is a phase II study with a limited sample size (about 50 patients per arm) and follow-up (median, 2.6 years). The study reflected the real-life practice of allowing heterogeneous diagnostic criteria for ET and included several patients still treated with HC in the BAT arm. Further studies with a longer follow-up could provide additional data on the role of ruxolitinib in ET, with particular regard to the unmet clinical need of prevention of disease transformation. In this regard, a recently updated long-term, open-label study in 39 ET patients resistant or intolerant to HC did not register any case of transformation to post-ET MF or AML after a median exposure to ruxolitinib of 6.8 years.7 The study demonstrated durable reduction in platelet and white blood cell counts and improvements in ET-related symptoms with ruxolitinib treatment.7 Overall, the MAJIC-ET trial provides a relevant piece of information for a better understanding of the optimal treatment of patients with ET who are resistant or intolerant to HC. Current guidelines recommend interferon or anagrelide as second-line therapy for ET,8 and these indications are confirmed by this study. At variance to patients with PV, ruxolitinib does not represent the first choice for most ET patients resistant or intolerant to HC, with the possible exception of those severely symptomatic, particularly for pruritus. Future studies of JAK2 inhibitors or other novel drugs in ET should further address the questions raised in this trial. Likewise, future randomized studies should include agents with different mechanisms of action reported in ET.9,10 The key issue is the indolent nature of this disease and to carefully balance the possible benefits, risks, and costs of overtreatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal