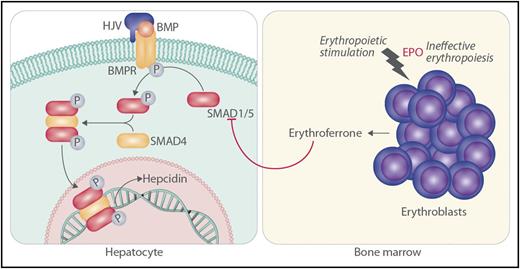
In this issue of Blood, Wang et al show that SMAD1 and SMAD5 act cooperatively to increase hepatic hepcidin expression in response to iron-mediated bone morphogenetic protein (BMP) signaling, and they provide evidence that erythroferrone produced by bone marrow progenitors may suppress hepatic hepcidin expression by inhibiting these same factors (see figure).1
The work of Wang et al is consistent with the following scenario. In the hepatocyte, SMAD1 and SMAD5 cooperate to mediate iron-related signaling through hemojuvelin (HJV), BMP, and BMP receptor (BMPR) for the promotion of hepcidin transcription through phosphorylated SMAD4. Homozygous inactivation of HJV or other molecules in the hepcidin-activation pathway leads to hemochromatosis through decreased SMAD1 and SMAD5 signaling and lack of hepcidin. Stimulation of erythroblasts by erythropoietin (EPO), especially in the setting of ineffective erythropoiesis, leads to increased production of erythroferrone by erythroblasts. In turn, erythroferrone serves to decrease phosphorylation (P) of SMAD1 and SMAD5 in hepatocytes, leading to iron loading as a result of lack of hepcidin.
The work of Wang et al is consistent with the following scenario. In the hepatocyte, SMAD1 and SMAD5 cooperate to mediate iron-related signaling through hemojuvelin (HJV), BMP, and BMP receptor (BMPR) for the promotion of hepcidin transcription through phosphorylated SMAD4. Homozygous inactivation of HJV or other molecules in the hepcidin-activation pathway leads to hemochromatosis through decreased SMAD1 and SMAD5 signaling and lack of hepcidin. Stimulation of erythroblasts by erythropoietin (EPO), especially in the setting of ineffective erythropoiesis, leads to increased production of erythroferrone by erythroblasts. In turn, erythroferrone serves to decrease phosphorylation (P) of SMAD1 and SMAD5 in hepatocytes, leading to iron loading as a result of lack of hepcidin.
How patients with anemias characterized by ineffective erythropoiesis develop systemic iron overload in the absence of blood transfusions is a fascinating question that hematologists have pondered for many years. Thalassemia major and intermedia syndromes are important examples of the association of the increased erythropoietic activity of intramedullary hemolysis with enhanced intestinal iron absorption.2 The magnitude of iron overload that may occur in conditions marked by ineffective erythropoiesis is independent of the degree of anemia,3 and the predominantly parenchymal iron loading in ineffective erythropoiesis is similar to hereditary hemochromatosis. These observations raised the possibility that the 2 conditions share in common a final pathophysiologic pathway.4 Indeed, this proved to be the case; it emerged over the past 15 years that hepcidin produced by hepatocytes has a central role in iron homeostasis and that deficiency of hepcidin with respect to the body’s iron burden underlies the iron loading seen in both hereditary hemochromatosis and anemias characterized by ineffective erythropoiesis.5
HFE, HJV encoding hemojuvelin, HAMP encoding hepcidin, and TFR2 encoding transferrin receptor 2 are genes of the hepcidin-activating pathway in hepatocytes; autosomal-recessive inactivation of any 1 of these genes leads to deficiency of hepcidin and systemic iron overload or hemochromatosis.5 Investigation of the function of these genes showed that BMP signaling through phosphorylation of SMAD proteins is a central pathway to regulate hepcidin transcription.6,7 In particular, previous research indicated that iron-related BMP signals lead to the phosphorylation of SMAD1, SMAD5, and SMAD8 and to the promotion of hepcidin transcription through the common mediator SMAD4.7,8 In this issue, Wang et al explored the individual contributions of SMAD1, SMAD5, and SMAD8 to hepcidin regulation. Knockdown of SMAD1 or SMAD5 but not SMAD8 inhibited hepcidin messenger RNA (mRNA) expression in Hep3B cells, so the investigators focused further on SMAD1 and SMAD5. They generated mice with hepatocyte-specific inactivation of Smad1, Smad5, or both to demonstrate that SMAD1 and SMAD5 have overlapping functions in regulating hepcidin expression but that the activity of both is necessary for optimal regulation.
Investigators recently discovered that erythroferrone, a factor secreted by erythroid progenitors in the bone marrow, is an important mediator of the suppression of hepatic hepcidin expression that is observed with increased erythropoiesis, especially ineffective erythropoiesis.9,10 Exactly how erythroferrone suppresses hepcidin expression and whether the mechanism involves the BMP-SMAD signaling pathway is uncertain. Wang et al found that erythropoietin robustly induced bone marrow erythroferrone mRNA in control mice and mice with hepatic inactivation of both Smad1 and Smad5 but suppressed liver hepcidin mRNA only in control mice. In keeping with this observation, erythropoietin and erythroferrone reduced the phosphorylation of SMAD1 and SMAD5 in parallel with decreasing the expression of hepcidin in the livers of control mice and in control Hep3B cells. Furthermore, erthroferrone failed to decrease hepcidin expression in hepatocytes with inactivation of Smad1 and Smad5 and in Hep3B cells with knockdown of SMAD1 and SMAD5. These observations are consistent with the possibility that erythroferrone acts through SMAD1 and SMAD5 signaling to suppress hepcidin production.
The report by Wang et al represents an important advance in our understanding of the details of BMP signaling in hepcidin regulation. The results indicate that SMAD1 and SMAD5, but not SMAD8, work cooperatively to control hepcidin expression. The evidence for a role of SMAD1 and SMAD5 in mediating hepatic hepcidin suppression in response to erythropoietin and in response to erythroferrone secreted by bone marrow erythroid progenitors is of particular interest to hematologists. Although more details need to be worked out, answers are now forthcoming to the old question of how ineffective erythropoiesis leads to nontransfusional iron overload.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal