Key Points
LPA converts monocytes into macrophages.
LPA mediates macrophage formation via Akt/mTor pathway; PPARγ is a master regulator of LPA-derived macrophages.
Abstract
Monocytes and macrophages represent critical arms of the innate immune system and are considered regulators and effectors of inflammation and the innate immune response. Monocytes can mobilize from bone marrow, traffic to their required destination, and differentiate into effector cells, depending on the local tissue environment, to perform multiple roles during infection or inflammation, making them important components of body’s immune defense. Macrophages have diverse roles in tissue homeostasis, development, and tissue repair following injury. Adult bone marrow monocytes can give rise to tissue-resident macrophages during infection or inflammatory reactions, besides self-replication of tissue resident macrophages. Lysophosphatidic acid (LPA), a lipid by-product of autotaxin activity, is involved in cancer, vascular defects, and neural tissue, but is largely unexplored in immune system. Here, we reveal an unexpected function of LPA that transfigures CD11b+ murine monocytes into F4/80+ macrophages. LPA-stimulated Akt/mTOR signaling is critical for LPA-mediated macrophage development in mice. Additionally, transcriptome analysis reveals that PPARγ is the key transcriptional regulator in the development of LPA-induced macrophages. In humans, LPA mediates macrophage formation following similar pathways. These findings identify a critical role for LPA in regulating innate immune system.
Introduction
Monocytes and macrophages constitute important components of the mononuclear phagocytic system and play diverse roles during infection, inflammation, and tissue injury and repair. Monocytes differentiate into macrophages following stimulation with cytokines and/or microbial molecules.1,2 Macrophages play a central role in the maintenance of tissue homeostasis, development, restoration after injury as well as the initiation and resolution of innate and adaptive immunity. Although macrophages were long considered to be derived from differentiation of bone marrow (BM) monocytes, recent studies have proved that tissue-resident macrophages are derived from yolk sac macrophages3 and fetal liver macrophages,4 can self-replicate from local proliferation,5 and do not solely depend on adult BM monocytes. However, during homeostatic adaptations, injury and inflammation macrophages of different phenotypes can be recruited from the monocyte reservoirs of blood, spleen, and BM.6,7 Lysophosphatidic acid (LPA), a bioactive phospholipid, exerts numerous cell responses, including cell motility, neuropathic pain, infertility, cardiovascular disease, inflammation, fibrosis, and cancer.8-10 This diversity is mediated by broad and overlapping expression patterns and multiple downstream signaling pathways activated by identical LPA receptors, but this lipid molecule remains little known in the immune system.9,11-14 Here we reveal that LPA, a small lipid molecule, converts monocytes into macrophages in both mice and humans.
Study design
Monocytes were isolated from the BM of C57BL/6 mice using negative selection of mouse monocytes (CD11b+) via magnetic beads from Stem Cell Technologies. Isolated BM monocytes were incubated in the presence of LPA, macrophage colony-stimulating factor (M-CSF), and Akt, mTor, or PPARγ inhibitors for the indicated periods and harvested for flow cytometry, gene expression, or immunoblotting analyses (for details, see supplemental Methods, available on the Blood Web site).
Results and discussion
To explore the role of LPA on monocytes, we added LPA on CD11b+ monocytes isolated from the BM of 6- to 8-week-old C57BL/6 mice and observed them after 5 days. To our surprise, we found that the monocytes had survived and phenotypically been converted into a different cell type. Giemsa staining revealed their morphology was similar to that of macrophages (Figure 1A-B). F4/80 is one of the most specific cell-surface markers for murine macrophages.15-18 Our immunoblot, immunofluorescence, and flow cytometry analysis showed F4/80 expression in these macrophages (Figure 1B-D; supplemental Figure 1E), confirming that these cells were macrophages. Quantitative polymerase chain reaction (qPCR) analysis showed increased expression of F4/80 and CD11b surface markers in a dose-dependent manner in LPA-treated monocytes. M-CSF, a well-known macrophage-inducing growth factor,19-21 was used as a positive control (Figure 1E-F; supplemental Figure 1A-B). Furthermore, LPA-treated monocytes did not show much enhancement in the proliferation of differentiating F4/80 macrophages (supplemental Figure 2D-E). To further check the effect of LPA on monocytes other than those of BM origin, we isolated and treated splenic monocytes with LPA and found that it induced macrophage formation in splenic-origin monocytes as well (supplemental Figure 1C-D).
LPA converts murine monocytes into macrophages via the Akt-mTor pathway. (A) Representative phase contrast images of mouse monocytes cultured in RPMI only (control [ctrl]) or in RPMI with LPA (10 µM) or M-CSF (10 ng/mL) for up to 7 days. (B) Upper panel: Giemsa-stained images of mouse monocytes cultured in vitro in the presence of LPA or M-CSF for 7 days. Figures are representative of 10 images (n = 10). Lower panel: Immunoblot showing levels of F4/80 and b-actin in control (RPMI), LPA, and M-CSF–treated monocytes. (C) FACS plots showing proportion of CD11b+ and F4/80+ macrophages in monocytes treated with LPA or M-CSF for 5 days. (D) Quantification of percentage of CD11b+ and F4/80+ cells in monocytes cultured in medium and cells cultured in medium with LPA or M-CSF. (E-F) Quantification of CD11b- and F4/80-relative mRNA in monocytes (control) and in monocytes cultured for 5 days in medium with different doses of LPA (50 and 10 μM) or M-CSF. (G-H) Immunoblot showing levels of p-Akt, p-mTor, Akt, and mTor in macrophages differentiated from mouse monocytes cultured in medium with LPA at indicated time (t) points. (I) Representative Giemsa-stained images of LPA-derived macrophages or M-CSF–derived macrophages without or with pretreatment of Akt inhibitor (LY-294002 [Ly]). (J) FACS plots showing the proportion of CD11b+ and F4/80+ macrophages in monocytes cultured in the presence of LPA, M-CSF, or LPS and monocytes pretreated with Akt inhibitor (LY-294002) and mTor inhibitor (rapamycin) and cultured in medium containing LPA, M-CSF, or LPS, respectively. (K) Quantification of CD11b+ and F4/80+ macrophages cultured in medium with only LPA and LPA after pretreatment with Akt or mTor inhibitor, respectively. Scale bars, 100 μm. Graph presents mean ± standard deviation (SD) of 5 experiments per condition. Error bars represent SD. Student t test was used for all statistical analyses (***P < .001, **P < .01, *P < .05).
LPA converts murine monocytes into macrophages via the Akt-mTor pathway. (A) Representative phase contrast images of mouse monocytes cultured in RPMI only (control [ctrl]) or in RPMI with LPA (10 µM) or M-CSF (10 ng/mL) for up to 7 days. (B) Upper panel: Giemsa-stained images of mouse monocytes cultured in vitro in the presence of LPA or M-CSF for 7 days. Figures are representative of 10 images (n = 10). Lower panel: Immunoblot showing levels of F4/80 and b-actin in control (RPMI), LPA, and M-CSF–treated monocytes. (C) FACS plots showing proportion of CD11b+ and F4/80+ macrophages in monocytes treated with LPA or M-CSF for 5 days. (D) Quantification of percentage of CD11b+ and F4/80+ cells in monocytes cultured in medium and cells cultured in medium with LPA or M-CSF. (E-F) Quantification of CD11b- and F4/80-relative mRNA in monocytes (control) and in monocytes cultured for 5 days in medium with different doses of LPA (50 and 10 μM) or M-CSF. (G-H) Immunoblot showing levels of p-Akt, p-mTor, Akt, and mTor in macrophages differentiated from mouse monocytes cultured in medium with LPA at indicated time (t) points. (I) Representative Giemsa-stained images of LPA-derived macrophages or M-CSF–derived macrophages without or with pretreatment of Akt inhibitor (LY-294002 [Ly]). (J) FACS plots showing the proportion of CD11b+ and F4/80+ macrophages in monocytes cultured in the presence of LPA, M-CSF, or LPS and monocytes pretreated with Akt inhibitor (LY-294002) and mTor inhibitor (rapamycin) and cultured in medium containing LPA, M-CSF, or LPS, respectively. (K) Quantification of CD11b+ and F4/80+ macrophages cultured in medium with only LPA and LPA after pretreatment with Akt or mTor inhibitor, respectively. Scale bars, 100 μm. Graph presents mean ± standard deviation (SD) of 5 experiments per condition. Error bars represent SD. Student t test was used for all statistical analyses (***P < .001, **P < .01, *P < .05).
Immunoblot analysis for phosphorylated proteins showed increased phosphorylation of Akt in monocytes after LPA was added, with further downstream mTor phosphorylations (Figure 1G-H; supplemental Figure 2A). To confirm the involvement of the Akt/mTor pathway in LPA-mediated macrophage formation, we pretreated mouse monocytes with a specific Akt inhibitor (LY294002) and added LPA to these monocytes to convert them into macrophages. Morphological observation indicated reduced formation of macrophages after Akt inhibitor pretreatment (Figure 1I; supplemental Figure 2B-C). FACS analysis showed a drastic reduction in the percentage of F4/80-expressing cells in LPA-treated monocytes, but did not affect macrophage formation from M-CSF or lipopolysaccharide (LPS) at a similar dose (Figure 1J-K). Mouse monocytes pretreated with mTor inhibitor (rapamycin) followed by the addition of LPA, M-CSF, or LPS showed no significant effect on M-CSF–mediated macrophage formation, whereas it inhibited macrophage formation in LPA-treated monocytes at a similar dose (Figure 1J; supplemental Figure 2C).
Thioglycollate injection in the pleural and peritoneum cavities elicits a higher number of monocytes from circulation.5,22,23 C57BL/6 mice were injected with 3% thioglycollate in the peritoneum and pleural cavities followed by LPA injection in the same cavities. The total cell population was isolated from the peritoneal and pleural cavities 3 days later and showed a higher percentage of F4/80-expressing cells in LPA-injected mice compared with phosphate-buffered saline–injected mice both in the peritoneum and pleural cavities (Figure 2A), confirming that LPA converts monocytes into macrophages in vivo.
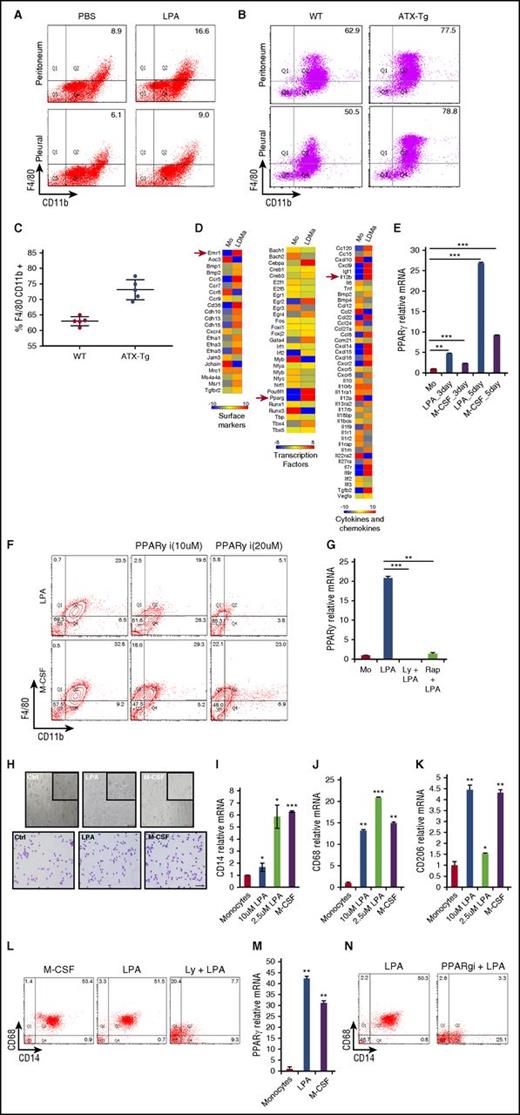
LPA generates macrophages in vivo and in humans through a common transcription factor, PPARγ, both in mice and humans. (A) FACS plots showing percentage of CD11b+ and F4/80+ macrophages elicited in peritoneal and pleural cavities of C57BL/6 mice injected with 3% thioglycollate in the absence or presence of 20 µg LPA. PBS, phosphate-buffered saline. (B) FACS plots showing percentage of CD11b+ and F4/80+ macrophages elicited in peritoneal and pleural cavities of FVB (FVB-WT) and FVB-ATX-Tg mice injected with 3% thioglycollate. (C) Proportion of peritoneal macrophages positive for F4/80 CD11b in FVB WT and FVB ATX-Tg mice. (D) Heat map showing the expression of surface markers, transcription factors, and cytokine-related genes in monocytes (Mo) and LPA-derived macrophages (LDMa). (E) Quantification of PPARγ mRNA in mouse monocytes and macrophages derived from LPA or M-CSF–treated mouse monocytes for different days. (F) FACS plots showing percentage of CD11b and F4/80 macrophages in monocytes pretreated with different doses of PPARγ inhibitor and cultured in the presence of LPA or M-CSF for 5 days. (G) Quantification of PPARγ in monocytes (control), monocytes treated with LPA, and monocytes pretreated with Akt inhibitor or mTor inhibitor and cultured further in medium supplemented with LPA for 5 days. Rap, rapamycin. (H) Representative phase contrast and Giemsa-stained images of human monocytes cultured in RPMI only (control) and human monocytes cultured in medium with 2.5 µM LPA and 10 ng/mL M-CSF for 7 days. (I-K) Quantification of CD14, CD68, and CD206 mRNA in human monocytes and macrophages derived from human monocytes cultured with different doses of LPA or M-CSF at day 5. (L) FACS plots showing percentage of CD14+ and CD68+ macrophages in monocytes treated with M-CSF, LPA, or Akt inhibitor (LY294002)-pretreated monocytes cultured in medium with LPA for 5 days. (M) Quantification of PPARγ-relative mRNA in human monocytes and monocytes cultured in medium with LPA or M-CSF for 7 days. (N) FACS plots showing percentage of CD14+ and CD68+ macrophages in human monocytes cultured with or without PPARγ inhibitor in the presence of LPA for 5 days. Graphs depict mean ± SD of 4 or 5 mice. Data are representative of 2 independent experiments. Error bars represent SD. Scale bars, 100 μm. Student t test was used for all statistical analyses (***P < .001).
LPA generates macrophages in vivo and in humans through a common transcription factor, PPARγ, both in mice and humans. (A) FACS plots showing percentage of CD11b+ and F4/80+ macrophages elicited in peritoneal and pleural cavities of C57BL/6 mice injected with 3% thioglycollate in the absence or presence of 20 µg LPA. PBS, phosphate-buffered saline. (B) FACS plots showing percentage of CD11b+ and F4/80+ macrophages elicited in peritoneal and pleural cavities of FVB (FVB-WT) and FVB-ATX-Tg mice injected with 3% thioglycollate. (C) Proportion of peritoneal macrophages positive for F4/80 CD11b in FVB WT and FVB ATX-Tg mice. (D) Heat map showing the expression of surface markers, transcription factors, and cytokine-related genes in monocytes (Mo) and LPA-derived macrophages (LDMa). (E) Quantification of PPARγ mRNA in mouse monocytes and macrophages derived from LPA or M-CSF–treated mouse monocytes for different days. (F) FACS plots showing percentage of CD11b and F4/80 macrophages in monocytes pretreated with different doses of PPARγ inhibitor and cultured in the presence of LPA or M-CSF for 5 days. (G) Quantification of PPARγ in monocytes (control), monocytes treated with LPA, and monocytes pretreated with Akt inhibitor or mTor inhibitor and cultured further in medium supplemented with LPA for 5 days. Rap, rapamycin. (H) Representative phase contrast and Giemsa-stained images of human monocytes cultured in RPMI only (control) and human monocytes cultured in medium with 2.5 µM LPA and 10 ng/mL M-CSF for 7 days. (I-K) Quantification of CD14, CD68, and CD206 mRNA in human monocytes and macrophages derived from human monocytes cultured with different doses of LPA or M-CSF at day 5. (L) FACS plots showing percentage of CD14+ and CD68+ macrophages in monocytes treated with M-CSF, LPA, or Akt inhibitor (LY294002)-pretreated monocytes cultured in medium with LPA for 5 days. (M) Quantification of PPARγ-relative mRNA in human monocytes and monocytes cultured in medium with LPA or M-CSF for 7 days. (N) FACS plots showing percentage of CD14+ and CD68+ macrophages in human monocytes cultured with or without PPARγ inhibitor in the presence of LPA for 5 days. Graphs depict mean ± SD of 4 or 5 mice. Data are representative of 2 independent experiments. Error bars represent SD. Scale bars, 100 μm. Student t test was used for all statistical analyses (***P < .001).
LPA is synthesized from lysophosphatidylcholine by the catalytic activity of autotaxin (Atx) in biological systems.9,24 To validate our finding with conditional Atx transgenic (ATX-Tg) mice, which produce more LPA because of a higher Atx expression in the circulation,25 we injected 3% thioglycollate in the peritoneal and pleural cavities of wild-type (FVB WT) and FVB ATX-Tg mice. Our analysis showed a higher percentage of F4/80-expressing cells in ATX-Tg mice compared with WT mice (Figure 2B-C).
RNA sequencing of isolated monocytes and LPA-converted macrophages was performed. Supplemental Figure 4A highlights a set of genes whose expression distinguishes between monocytes and LPA-mediated macrophages; 6 biological categories with the predicted functionality of each subset are spotlighted (Figure 2D; supplemental Figure 4B-E). F4/80 (EMR1) was highly expressed in LPA-converted macrophages, along with PPARγ transcription factor (TF) as revealed by network analysis suggesting PPARγ is a “master” TF for LPA-mediated macrophages (supplemental Figure 4F).
qPCR confirmed higher expression of PPARγ along with macrophage-specific TFs (Figure 2E; supplemental Figure 5A-B). Further, chromatin immunoprecipitation-qPCR analysis revealed measurable recruitment of endogenous PPARγ at the PPARγ-binding sites26 in LPA-derived macrophages compared with control monocytes (supplemental Figure 5C). PPARγ inhibition drastically reduced the macrophage population (Figure 2F; supplemental Figure 6A). Interestingly, Akt or mTor inhibitor pretreatment reduced PPARγ expression during LPA-mediated monocyte differentiation to macrophages (Figure 2G). To check the functional implication of these macrophages, we stimulated LPA-derived macrophages with LPS and found elevated levels of cytokines and chemokine secretion similar to M-CSF–derived macrophages (supplemental Figure 7A-F).
To determine whether murine development of macrophages from monocytes were coherent with the LPA effect on human monocytes, CD14+ monocytes isolated from human peripheral blood mononuclear cells were treated with different doses of LPA. These LPA-generated macrophages showed a higher expression of CD68, as seen by flow cytometry and CD14, CD64, CD68, and CD206 via qPCR, comparable to human M-CSF–generated macrophages (Figure 2H-L; supplemental Figure 6B). Further qPCR showed that 2.5 µM LPA was optimum for human macrophage formation, with higher expression of CD68, a macrophage marker, and CD14 (supplemental Figure 9A-B). To further confirm the Akt/mTor pathway found in LPA-mediated mouse macrophage formation in human macrophage formation from CD14 monocytes, we pretreated CD14 monocytes with Akt inhibitor followed by the addition of LPA. We found that Akt inhibitor–treated CD14 monocytes severely reduced the percentage of macrophages on further LPA treatments (Figure 2L). qPCR analysis also confirmed enhanced expression of PPARγ messenger RNA (mRNA) in human monocytes. Monocytes pretreated with PPARγ inhibitor did not form macrophages from CD14 monocytes in the presence of LPA, confirming its role in human macrophage formation (Figure 2M-N; supplemental Figure 8A-B) and establishing PPARγ as the key master regulator in LPA-mediated macrophage development.
This work demonstrates the unique potential of LPA to produce macrophages ex vivo and in vivo and in both mice and humans. Macrophages are an important component of the innate immune system and produce a diverse range of biologically active molecules that participate in both beneficial and detrimental outcomes in inflammation. As a result, this makes LPA-mediated macrophages an important avenue for therapeutic interventions, and their products may open ways for controlling inflammatory diseases.16,22 Akt-mTor or PPARγ is critical in LPA-mediated macrophage formation and differentiation as seen in vitro and in vivo. Furthermore, transcriptome analysis characterizes these new LPA-mediated macrophages.
This finding may ultimately provide a foundation for LPA in the innate system. Interestingly, in many types of cancer and tumors, LPA is highly upregulated.9,14,27 This work facilitates a future possible role of LPA in tumor-associated macrophages, but a better understanding is needed of the underlying mechanisms for this reprogramming.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gordon Mills, MD Anderson Cancer Center, Houston, TX, for generously sharing ATX transgenic mice; Khokan Rana for his assistance in the animal studies; and Bioserve Biotechnologies, India, for help in sequencing and data analysis.
This work was supported by the Department of Biotechnology, New Delhi (grant 102/IFD/SAN/2237) and the Institute of Life Sciences core fund.
Authorship
Contribution: R.R. contributed to project planning, experimental work, data analysis, and writing the manuscript; and V.R. contributed to project planning, data analysis, writing the manuscript, and overall supervision of this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vivek Rai, Institute of Life Sciences (An Autonomous Institute of Department of Biotechnology New Delhi), Bhubaneswar-751023, India; e-mail: vivekrai.a@gmail.com.

![Figure 1. LPA converts murine monocytes into macrophages via the Akt-mTor pathway. (A) Representative phase contrast images of mouse monocytes cultured in RPMI only (control [ctrl]) or in RPMI with LPA (10 µM) or M-CSF (10 ng/mL) for up to 7 days. (B) Upper panel: Giemsa-stained images of mouse monocytes cultured in vitro in the presence of LPA or M-CSF for 7 days. Figures are representative of 10 images (n = 10). Lower panel: Immunoblot showing levels of F4/80 and b-actin in control (RPMI), LPA, and M-CSF–treated monocytes. (C) FACS plots showing proportion of CD11b+ and F4/80+ macrophages in monocytes treated with LPA or M-CSF for 5 days. (D) Quantification of percentage of CD11b+ and F4/80+ cells in monocytes cultured in medium and cells cultured in medium with LPA or M-CSF. (E-F) Quantification of CD11b- and F4/80-relative mRNA in monocytes (control) and in monocytes cultured for 5 days in medium with different doses of LPA (50 and 10 μM) or M-CSF. (G-H) Immunoblot showing levels of p-Akt, p-mTor, Akt, and mTor in macrophages differentiated from mouse monocytes cultured in medium with LPA at indicated time (t) points. (I) Representative Giemsa-stained images of LPA-derived macrophages or M-CSF–derived macrophages without or with pretreatment of Akt inhibitor (LY-294002 [Ly]). (J) FACS plots showing the proportion of CD11b+ and F4/80+ macrophages in monocytes cultured in the presence of LPA, M-CSF, or LPS and monocytes pretreated with Akt inhibitor (LY-294002) and mTor inhibitor (rapamycin) and cultured in medium containing LPA, M-CSF, or LPS, respectively. (K) Quantification of CD11b+ and F4/80+ macrophages cultured in medium with only LPA and LPA after pretreatment with Akt or mTor inhibitor, respectively. Scale bars, 100 μm. Graph presents mean ± standard deviation (SD) of 5 experiments per condition. Error bars represent SD. Student t test was used for all statistical analyses (***P < .001, **P < .01, *P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/9/10.1182_blood-2016-10-743757/5/m_blood743757f1.jpeg?Expires=1769110230&Signature=K53a-nKAJXIESjLmvA4YYIjSGVACM8MNKyUWgBVKPljaWybcsz~CR-XiYoEBVJPJGkWFcHh5ZDIJvfWYuFJRUgVWclITxJbuuqeLPX1fEvHDzNIcGfqtH2VF3zEcG7OVB1ozXdJnkDBuMjuNEYTX4-aDwDej1JnLp14D2DET-re9JtpcRoFxbD9SMAgv4Jw0zjRLScSo~BukL-F7VpsMYIb8fOs8fJkm9aIct6B~7KjUfiq8iBfbmlukxgBGw294A5rjOrGxXFHsxs6hiBw28tQRjuENRNbdtbdrt7aFL8-6o3NifU6KRcjIBiv-ABw5s25gd00rd9nrUKZMDxrXDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)