Key Points
SCT status is not significantly associated with longitudinal changes in fitness among African Americans.
SCT status is not an independent risk factor for hypertension, diabetes, or metabolic syndrome among African Americans.
Abstract
The contribution of sickle cell trait (SCT) to racial disparities in cardiopulmonary fitness is not known, despite concerns that SCT is associated with exertion-related sudden death. We evaluated the association of SCT status with cross-sectional and longitudinal changes in fitness and risk for hypertension, diabetes, and metabolic syndrome over the course of 25 years among 1995 African Americans (56% women, 18-30 years old) in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Overall, the prevalence of SCT was 6.8% (136/1995) in CARDIA, and over the course of 25 years, 46% (738/1590), 18% (288/1631), and 40% (645/1,611) of all participants developed hypertension, diabetes, and metabolic syndrome, respectively. Compared with participants without SCT, participants with SCT had similar baseline measures of fitness in cross-section, including exercise duration (535 vs 540 seconds; P = .62), estimated metabolic equivalent of tasks (METs; 11.6 vs 11.7; P = .80), maximum heart rate (174 vs 175 beats/min; P = .41), and heart rate at 2 minutes recovery (44 vs 43 beats/min; P = .28). In our secondary analysis, there was neither an association of SCT status with longitudinal changes in fitness nor an association with development of hypertension, diabetes, or metabolic syndrome after adjustment for sex, baseline age, body mass index, fitness, and physical activity. SCT is not associated with reduced fitness in this longitudinal study of young African American adults, suggesting the increased risk for exertion-related sudden death in SCT carriers is unlikely related to fitness. SCT status also is not an independent risk factor for developing hypertension, diabetes, or metabolic syndrome.
Introduction
Sickle cell trait (SCT) is caused by the inheritance of a single copy of the variant β globin gene, which results in production of abnormal sickle hemoglobin. The coinheritance of 2 copies of the abnormal gene results in sickle cell anemia (SCA), a clinically significant blood disorder seen primarily among individuals of African or Afro-Caribbean descent. Historically, SCT, which is observed in 8% of the African American population in the United States, has been considered a clinically benign condition. Recent studies, however, suggest SCT may confer a higher risk for chronic kidney disease, thrombosis, stroke, and pregnancy-related complications in its carriers.1-6 Importantly, there is growing concern SCT may be associated with sudden death during strenuous physical activity and exercise, leading to the National College Athletics Association to mandate carrier status testing among all student athletes in division I institutions in 2010, and later in all division II and III institutions.7-9
Growing evidence suggests SCT carriers may exhibit abnormal physiologic responses to cardiopulmonary exercise testing when compared with controls without SCT.10-13 More recently, SCT status was also shown to be associated with a significantly higher risk for exertional rhabdomyolysis among black soldiers on active duty in the US Army.14 However, findings relating to the relationship between SCT status and cardiopulmonary fitness in these studies have been equivocal, in part limited by small sample sizes. Also, the effect of SCT on the development of other cardiovascular risk factors, as well as how this relationship is influenced by fitness, has not been previously examined despite growing evidence for an increased risk for chronic kidney disease in SCT carriers. The Coronary Artery Risk Development in Young Adults (CARDIA) study and its genetic repository provide a unique opportunity to study fitness and cardiovascular risk factors in a large cohort of young African Americans who are followed for up to 25 years. Thus, we tested the hypothesis that SCT carrier status was associated with lower fitness at baseline and worsening fitness over follow-up. Given the association between poor fitness and development of cardiovascular disease risk factors, we further hypothesized that SCT carriers would be at increased risk of developing hypertension, diabetes, and metabolic syndrome.
Methods
Participant selection
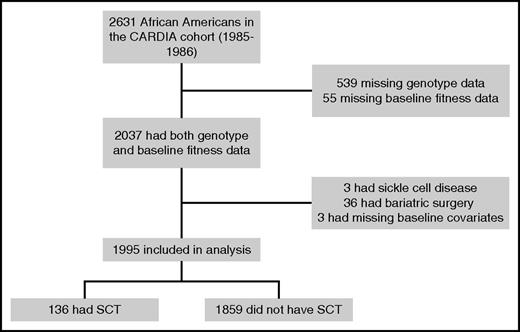
Study participants were from CARDIA, a multicenter, longitudinal study of the development of coronary artery disease risk factors in young adults. Details of the CARDIA study design and inclusion/exclusion criteria have been previously published.15 Between 1985 and 1986, 5115 African American and white adults (52% African American, 55% women), aged 18 to 30 years, were recruited from 4 US urban areas (Birmingham, Alabama; Oakland, California; Chicago, Illinois; and Minneapolis, Minnesota). Institutional review board approval was obtained at each participating center. Informed consent was obtained from all participants. Seven follow-up examinations (at years 2, 5, 7, 10, 15, 20, and 25) have been completed since enrollment. Our study included 2631 self-reported African American, nonpregnant women and men, of whom 2037 had available SCT genotype (n = 539 missing) and fitness data at baseline (n = 55 missing). We excluded participants with sickle cell disease (n = 3), participants who underwent bariatric surgery (n = 36), and participants with missing baseline covariates of interest (n = 3). The final cohort for analysis included 1995 African American participants (Figure 1).
Genotyping
For our analysis, we used available DNA samples from year 10 of follow-up, which was the first year DNA collection/storage was performed in the CARDIA study, or beyond. We performed single-gene, single-nucleotide polymorphism (SNP) genotyping by functionally tested TaqMan SNP Genotyping Assays in accordance with manufacturer protocols (Life Technologies, Grand Island, NY). Details about assay design and experimental procedures are described in Naik et al.16 We obtained genotype data for SNP rs334 encoding for hemoglobin S trait, otherwise known as SCT, and for SNP rs33930165 encoding for hemoglobin C trait, another common hemoglobin variant. We genotyped for hemoglobin C trait, given the possibility of coinheritance of hemoglobin S and C in some individuals, which results in a clinically significant variant of sickle cell disease. For quality control, no more than 3% replicate samples were included to evaluate the reproducibility of the genotyped data. Replicate samples were randomly distributed throughout the genotyping plate, set with a minimum of 2 replicates per plate. Genotyping concordance between the replicate pairs was 100% for both SNPs.
Cardiopulmonary fitness assessment
Fitness data were measured at baseline and again at years 7 and 20. Fitness was assessed using a graded, symptom-limited maximal treadmill test according to a modified Balke protocol, which included up to 9 stages (2 minutes each) of increasing difficulty defined by treadmill speed and incline. The modified Balke treadmill protocol is commonly used and is considered a safe and reliable approach to testing fitness.17 Participants were encouraged to exercise to maximal exertion, followed by a 2-minute recovery stage. The rate of energy expenditure for the completion of each stage of the test was estimated as previously described and was reported in metabolic equivalents (METs), where each MET represents the rate of energy expenditure equal to an oxygen consumption rate of 3.5 mL/kg/min.18 Heart rate (HR) and blood pressure were measured at rest and at end of each stage. HR recovery was defined as the difference between maximum HR and HR at 2 minutes into the recovery period.
Other measurements
A standardized protocol was followed across all field centers and at each examination to measure seated blood pressure, cholesterol, height, weight, waist circumference, and physical activity in participants after a 12-hour fast. Demographic characteristics, participation in physical activity, and medication use were obtained by interviewer-administered questionnaire. Body mass index (BMI) was obtained using a balanced beam scale and a vertical ruler for obtaining height and weight, respectively, and then calculated by dividing the weight in kilograms by the square of the height in meters. Resting blood pressure was measured 3 times at each examination, and the average of the last 2 measurements was used. Physical activity was assessed by a standard instrument, and energy expenditure for all moderate and vigorous activities was calculated in exercise units.19 Venous blood samples were obtained from participants. Plasma triglycerides and high-density lipoprotein (HDL) cholesterol levels were determined using an enzymatic assay by Northwest Lipids Research Laboratory (Seattle, Washington). Glucose was measured by the hexokinase method.
Cardiovascular disease risk factors
Diabetes was defined as fasting glucose ≥126 mg/dL, hemoglobin A1c >6.5%, postload glucose ≥200 mg/dL, or taking diabetes medication. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive medication use. Metabolic syndrome was defined as the presence of 3 or more of the following components: increased waist circumference (>102 cm in men and >88 cm in women), triglycerides ≥150 mg/dL or taking cholesterol-lowering medication, reduced HDL-C (<40 mg/dL in men and <50 mg/dL in women) or taking cholesterol-lowering medication, blood pressure >130/85 mm Hg or taking blood pressure medication, and fasting glucose ≥100 mg/dL or taking diabetes medication.20 Incidence of each CVD risk factor was identified at any follow-up examination among participants who were free from that condition at baseline.
Statistical analysis
Baseline characteristics were compared between participants with and without SCT, using Student's t test, χ2 or Fisher’s exact test, or Wilcoxon test as appropriate. For cross-sectional analyses, multiple linear regression analyses were used to evaluate the associations of SCT status and each continuous cardiopulmonary fitness measure (ie, fitness duration, estimated METs, maximum heart rate, and HR recovery at 2 minutes) separately, adjusted for sex, age, baseline physical activity, and BMI. To compare longitudinal change in each fitness measure between participants with and without SCT, we conducted a repeated measures analysis of covariance, using a mixed model approach in which within-subject correlations were modeled with a subject-specific random effect.21 For each model, the dependent variable was the time-dependent fitness measure of interest (at baseline, years 7 and 20). Time (defined as the years from baseline) was treated as a continuous variable, whereas SCT was treated as indicator variable, with no SCT as a referent. We included the cross-product term of SCT and time, with the β coefficient for the cross-product term indicating the magnitude of difference in average annual changes of each fitness measure relative to the no SCT group. All models were adjusted for sex, baseline age, baseline physical activity, and BMI.
Cox proportional hazards models were used to estimate the hazard ratios, and 95% confidence intervals (CIs) associated with developing each cardiovascular risk factor (hypertension, diabetes, metabolic syndrome) by SCT status, adjusting for sex, baseline age, BMI, fitness duration, and physical activity. Among nonpregnant women and men who had at least 4 follow-up examinations (n = 1643), we defined the incident development of each risk factor if the risk factor were identified at any of the follow-up examinations (at years 2, 5, 7, 10, 15, 20, or 25) among participants free from that risk factor at baseline. The time at risk was calculated from the baseline examination through the examination at which the risk factor was first identified, the year 25 examination, or the last examination before loss to follow-up, whichever occurred first. Analyses were conducted with SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). P value <.05 was considered statistically significant.
Results
Baseline characteristics of participants
Figure 1 illustrates the derivation of the final sample used in our analysis from the initial cohort. Of the remaining 1995 participants, 7% were determined to have SCT. According to baseline data, participants who had missing data were younger and had less education than participants included in the study sample. There was no difference in baseline prevalence of cardiovascular risk factors for participants with missing data vs those included in the sample. The mean age at the time of assessment was 24.8 and 24.3 years for participants with and without SCT, respectively. At baseline, diastolic blood pressure was slightly higher in participants with SCT (71 vs 69 mm Hg; P = .02), although the prevalence of hypertension, diabetes, and metabolic syndrome was similar between the 2 groups (Table 1). We found no significant difference in baseline fitness parameters in participants by SCT carrier status, even after adjusting for age, sex, reported physical activity, and BMI (Table 2). Adjusted mean duration of exercise (535 vs 540 seconds; P = .62) was similar in participants with SCT vs no SCT, as were adjusted estimated METs (11.6 vs 11.7 units; P = .80), maximum heart rate achieved (174 vs 175 bpm; P = .41), and heart rate at 2 minutes of recovery (44 vs 43 bpm; P = .28).
Baseline characteristics of African American participants by sickle cell trait status, 1985-1986
| Variable . | Total . | No SCT . | SCT . | P value* . |
|---|---|---|---|---|
| No., %† | 1995 | 1859 (93.2) | 136 (6.8) | |
| Age, years | 24.3 (3.8) | 24.3 (3.8) | 24.8 (3.9) | .17 |
| Male, % | 44.0 | 44.1 | 42.7 | .75 |
| BMI, kg/m2 | 25.2 (5.4) | 25.2 (5.5) | 24.7 (4.8) | .27 |
| Waist circumference, cm | 78.2 (11.7) | 78.3 (11.8) | 77.1 (10.7) | .23 |
| Hypertension, % | 3.1 | 3.1 | 2.9 | .93 |
| Systolic BP, mm Hg | 111.4 (10.8) | 111.3 (10.9) | 111.7 (10.3) | .70 |
| Diastolic BP, mm Hg | 68.8 (9.9) | 68.7 (9.9) | 70.7 (10.1) | .02 |
| Triglyceride, mg/dL (median [IQR]) | 58.0 (43.0-78.0) | 58.0 (43.0-78.0) | 57.0 (43.0-79.0) | .80 |
| LDL cholesterol, mg/dL | 110.3 (32.1) | 110.5 (32.1) | 108.2 (31.9) | .44 |
| HDL cholesterol, mg/dL | 54.4 (13.1) | 54.3 (13.1) | 55.2 (13.6) | .46 |
| Diabetes, % | 0.8 | 0.8 | 0.0 | .29 |
| Fasting glucose, mg/dL | 81.8 (15.6) | 81.9 (16.0) | 79.9 (8.1) | .14 |
| Metabolic syndrome, % | 2.0 | 2.0 | 2.2 | .93 |
| Total physical activity, EU (median [IQR]) | 316.0 (159.0-537.0) | 317.0 (162.0-537.0) | 294.0 (130.0-525.0) | .55 |
| Variable . | Total . | No SCT . | SCT . | P value* . |
|---|---|---|---|---|
| No., %† | 1995 | 1859 (93.2) | 136 (6.8) | |
| Age, years | 24.3 (3.8) | 24.3 (3.8) | 24.8 (3.9) | .17 |
| Male, % | 44.0 | 44.1 | 42.7 | .75 |
| BMI, kg/m2 | 25.2 (5.4) | 25.2 (5.5) | 24.7 (4.8) | .27 |
| Waist circumference, cm | 78.2 (11.7) | 78.3 (11.8) | 77.1 (10.7) | .23 |
| Hypertension, % | 3.1 | 3.1 | 2.9 | .93 |
| Systolic BP, mm Hg | 111.4 (10.8) | 111.3 (10.9) | 111.7 (10.3) | .70 |
| Diastolic BP, mm Hg | 68.8 (9.9) | 68.7 (9.9) | 70.7 (10.1) | .02 |
| Triglyceride, mg/dL (median [IQR]) | 58.0 (43.0-78.0) | 58.0 (43.0-78.0) | 57.0 (43.0-79.0) | .80 |
| LDL cholesterol, mg/dL | 110.3 (32.1) | 110.5 (32.1) | 108.2 (31.9) | .44 |
| HDL cholesterol, mg/dL | 54.4 (13.1) | 54.3 (13.1) | 55.2 (13.6) | .46 |
| Diabetes, % | 0.8 | 0.8 | 0.0 | .29 |
| Fasting glucose, mg/dL | 81.8 (15.6) | 81.9 (16.0) | 79.9 (8.1) | .14 |
| Metabolic syndrome, % | 2.0 | 2.0 | 2.2 | .93 |
| Total physical activity, EU (median [IQR]) | 316.0 (159.0-537.0) | 317.0 (162.0-537.0) | 294.0 (130.0-525.0) | .55 |
Values are presented as means (SD) unless otherwise indicated.
EU, exercise units; IQR, interquartile range (Q1-Q3); LDL, low-density lipoprotein.
P values for comparisons between SCT and No SCT (reference).
May vary for some variables.
Baseline graded exercise performance by sickle cell trait status
| . | Adjusted mean (95% CI) . | ||
|---|---|---|---|
| No SCT . | SCT . | P value* . | |
| No. | 1859 | 136 | |
| Duration, sec | 539.9 (535.1-544.6) | 535.2 (517.6-552.8) | .62 |
| Estimated METs | 11.7 (11.6-11.8) | 11.6 (11.4-11.9) | .80 |
| Maximum heart rate, beats/min | 175.1 (174.3-175.8) | 173.8 (171.0-176.7) | .41 |
| HR recovery at 2 min, beats/min | 43.3 (42.8-43.8) | 44.3 (42.5-46.2) | .28 |
| . | Adjusted mean (95% CI) . | ||
|---|---|---|---|
| No SCT . | SCT . | P value* . | |
| No. | 1859 | 136 | |
| Duration, sec | 539.9 (535.1-544.6) | 535.2 (517.6-552.8) | .62 |
| Estimated METs | 11.7 (11.6-11.8) | 11.6 (11.4-11.9) | .80 |
| Maximum heart rate, beats/min | 175.1 (174.3-175.8) | 173.8 (171.0-176.7) | .41 |
| HR recovery at 2 min, beats/min | 43.3 (42.8-43.8) | 44.3 (42.5-46.2) | .28 |
Models adjusted for age, sex, baseline physical activity, and BMI.
P values for comparisons between SCT and No SCT (reference).
Longitudinal changes in fitness and incident cardiovascular risk factors
We found no significant difference in annual change in graded exercise performance, including test duration, estimated METs, maximal heart rate achieved, or heart rate at 2 minutes of recovery between participants with and without SCT (Table 3). After adjusting for sex, baseline age, BMI, and physical activity, there was no significant association of SCT status with incident hypertension (hazard ratio, 1.22; 95% CI, 0.91-1.65; P = .19), diabetes (hazard ratio, 1.48; 95% CI, 0.96-2.27; P = .08), or metabolic syndrome (hazard ratio, 1.26; 95% CI, 0.92-1.74; P = .15) over the course of 25 years (Table 4).
Average annual change in graded exercise performance over the course of 20 years by sickle cell trait status
| . | No. . | Baseline mean (95% CI) . | Mean change/year (95% CI) . | Mean change/year (95% CI), relative to No SCT . | P value* . |
|---|---|---|---|---|---|
| Test duration , sec | |||||
| No SCT | 1402 | 540.4 (535.0-545.8) | −9.12 (−9.50 to −8.75) | Reference | |
| SCT | 101 | 540.8 (520.6-561.1) | −8.71 (−10.10 to −7.32) | 0.41 (−1.03 to 1.85) | .58 |
| Estimated METs | |||||
| No SCT | 1402 | 11.7 (11.6-11.8) | −0.12 (−0.13 to −0.11) | Reference | |
| SCT | 101 | 11.8 (11.5-12.1) | −0.12 (−0.15 to −0.10) | 0.00 (−0.03 to 0.02) | .82 |
| Maximum HR, beats/min | |||||
| No SCT | 1402 | 176.1 (175.2-177.0) | −0.56 (−0.61 to −0.51) | Reference | |
| SCT | 101 | 174.7 (171.4-177.9) | −0.46 (−0.71 to −0.20) | 0.10 (−0.10 to 0.30) | .34 |
| HR recovery at 2 min, beats/min | |||||
| No SCT | 1390 | 43.2 (42.6-43.8) | −0.25 (−0.28 to −0.21) | Reference | |
| SCT | 101 | 44.5 (42.3-46.6) | −0.24 (−0.39 to −0.09) | 0.01 (−0.15 to 0.16) | .94 |
| . | No. . | Baseline mean (95% CI) . | Mean change/year (95% CI) . | Mean change/year (95% CI), relative to No SCT . | P value* . |
|---|---|---|---|---|---|
| Test duration , sec | |||||
| No SCT | 1402 | 540.4 (535.0-545.8) | −9.12 (−9.50 to −8.75) | Reference | |
| SCT | 101 | 540.8 (520.6-561.1) | −8.71 (−10.10 to −7.32) | 0.41 (−1.03 to 1.85) | .58 |
| Estimated METs | |||||
| No SCT | 1402 | 11.7 (11.6-11.8) | −0.12 (−0.13 to −0.11) | Reference | |
| SCT | 101 | 11.8 (11.5-12.1) | −0.12 (−0.15 to −0.10) | 0.00 (−0.03 to 0.02) | .82 |
| Maximum HR, beats/min | |||||
| No SCT | 1402 | 176.1 (175.2-177.0) | −0.56 (−0.61 to −0.51) | Reference | |
| SCT | 101 | 174.7 (171.4-177.9) | −0.46 (−0.71 to −0.20) | 0.10 (−0.10 to 0.30) | .34 |
| HR recovery at 2 min, beats/min | |||||
| No SCT | 1390 | 43.2 (42.6-43.8) | −0.25 (−0.28 to −0.21) | Reference | |
| SCT | 101 | 44.5 (42.3-46.6) | −0.24 (−0.39 to −0.09) | 0.01 (−0.15 to 0.16) | .94 |
Fitness testing was performed at years 7 and 20. Analysis sample included participants with fitness data available at baseline and at least 1 more exam at year 7 or year 20. Models were adjusted for sex, baseline age, physical activity, and BMI.
SCT, sickle cell trait carrier status.
P values for comparisons between SCT and No SCT (reference).
Sickle cell trait and incident cardiovascular disease risk factors over the course of 25 years (hazard ratios)
| . | Hazard ratios . | |
|---|---|---|
| No./Total (%) . | Hazard ratio (95% CI) . | |
| Incident hypertension | ||
| No SCT | 682/1482 (56.9) | Reference |
| SCT | 56/108 (60.6) | 1.22 (0.91-1.65) |
| Incident diabetes | ||
| No SCT | 262/1519 (17.3) | Reference |
| SCT | 26/112 (23.2) | 1.48 (0.96-2.27) |
| Incident metabolic syndrome | ||
| No SCT | 596/1502 (39.7) | Reference |
| SCT | 49/109 (45.0) | 1.26 (0.92-1.74) |
| . | Hazard ratios . | |
|---|---|---|
| No./Total (%) . | Hazard ratio (95% CI) . | |
| Incident hypertension | ||
| No SCT | 682/1482 (56.9) | Reference |
| SCT | 56/108 (60.6) | 1.22 (0.91-1.65) |
| Incident diabetes | ||
| No SCT | 262/1519 (17.3) | Reference |
| SCT | 26/112 (23.2) | 1.48 (0.96-2.27) |
| Incident metabolic syndrome | ||
| No SCT | 596/1502 (39.7) | Reference |
| SCT | 49/109 (45.0) | 1.26 (0.92-1.74) |
Hazard ratio derived from Cox proportional hazards model. Incidence of each CVD risk factor was identified at any follow-up examination up to year 25. Models were adjusted for sex, baseline age, BMI, fitness (duration), and physical activity.
Discussion
To our knowledge, our study is the first to evaluate the influence of SCT status on long-term changes in fitness and development of cardiovascular disease risk factors. The 7% prevalence of SCT in the CARDIA cohort is similar to the estimated carrier rate in the United States. SCT had no significant effect on either baseline or annual changes in fitness, as estimated using multiple measures from a graded treadmill test. We also did not see between group differences in heart rate recovery at 2 minutes at baseline or over time, a surrogate marker for autonomic nervous system function. Baseline and 25-year incidence of hypertension, diabetes, and metabolic syndrome were not affected by SCT status.
Our findings, although largely null, have important implications for increasing our understanding of fitness and SCT. Existing data, both physiologic and epidemiologic, support the plausibility of an association between reduced fitness and SCT and provide the rationale for testing our primary hypothesis. Lower maximal oxygen consumption and workload have been reported in early small studies of African American males with SCT.22 Several studies have also sought to determine the pathophysiologic basis for the exertion-related sudden death described among individuals with SCT in case reports.23-26 In a recent population study of black soldiers on active duty in the US Army, SCT was associated with an adjusted risk for exertional rhabdomyolysis that was 54% higher than that associated with absence of SCT, although all-cause mortality was not affected by SCT status.14 Specific derangements associated with exercise challenge in SCT have included increased blood viscosity, endothelial activation, autonomic nervous system imbalance, and impairments in oxygen uptake kinetics.10,13,27-31 In contrast, our results do not demonstrate differences in baseline or longitudinal changes in fitness by SCT status, which is consistent with other studies that have also shown no difference in maximal oxygen uptake or key determinants of fitness, including lactate production and ventilatory threshold, in individuals with and without SCT.32-35 We also found no difference in autonomic nervous system function by SCT status, using our measure of heart rate recovery after exercise. However, variability in methodology used to define fitness and autonomic nervous system function may explain discrepancies in findings across studies.
Our findings suggest that SCT status alone probably does not explain the racial disparities in fitness observed between African Americans and non-African Americans, given that we found no difference in fitness by SCT status among African Americans in this cohort. That we did not find a difference using data from the CARDIA study is particularly important, given the young age of the cohort at the start of the study and the frequent description of exertion-related sudden death in young student athletes with SCT.7 Data from large-scale observational studies, including the National Health and Nutrition Examination Survey and Health, Risk Factors, Exercise Training and Genetics studies, have demonstrated lower fitness, measured directly by maximal tests or estimated from submaximal tests, in African American individuals when compared with their Caucasian counterparts.36,37 However, it is more likely that lower levels of physical activity and higher BMI, which may affect muscle oxidative potential, contribute to disparities in fitness between African Americans and Caucasians.37-40
At baseline, participants with SCT in the CARDIA study had a slightly, but significantly, higher diastolic blood pressure compared with those without SCT. However, the absolute difference was small, and the baseline prevalence of hypertension was not higher among participants with SCT. Our finding that SCT carriers do not have an increased risk of developing hypertension over the course of 25 years aligns with recent findings by Naik et al, who reported that SCT is associated with an increased risk for incident chronic kidney disease, decline in kidney function over time, and albuminuria in their study of almost 16 000 African American participants from various cardiovascular cohorts, including a subset of CARDIA participants.16 The authors, however, did not find baseline differences in hypertension by SCT status or that the association between SCT and chronic kidney disease was modified by hypertension. In our analysis, we focused on the relationship of SCT status to hypertension, but not chronic kidney disease, as hypertension is better tied to fitness as a predictor of cardiovascular disease risk. Further, we did not find that SCT status contributed to the incidence of diabetes or metabolic syndrome, despite their known relationship to fitness and the known increased risk for both cardiovascular risk factors among the general African American population.41 A strength of this study was that we adjusted our analysis of the association between SCT and development of cardiovascular risk factors by sex, baseline age, BMI, and baseline physical activity.
There are some limitations to our study that warrant discussion. The relatively low prevalence of SCT carriers in our cohort and the number of participants excluded as a result of missing genotype data may have limited our ability to detect significant differences in our primary outcomes. The CARDIA study was not powered to test the hypotheses in this analysis. Thus, sample size limitations could explain our null findings, especially for our assessment of the development of cardiovascular risk factors over time. However, our analysis used data from 1 of the most comprehensive, ongoing studies of fitness and cardiovascular disease risk and represents an important initial effort to look at the association between SCT and these factors. We could not examine mortality as an endpoint in our analysis of SCT carriers, as the participants in CARDIA are relatively young and there were very few deaths from chronic diseases. We also could not evaluate the effect of SCT on long-term changes in fitness past 20 years, given the limited follow-up in this study at the time of our analysis. Still, it was reasonable instead to assess differences in development of cardiovascular risk factors, considered a general surrogate marker for early mortality, in SCT carriers. In addition, fitness in CARDIA was primarily measured by duration of time on the treadmill. Breath-by-breath gas exchange was not performed to enable direct measurement of peak oxygen consumption during exercise, considered the reference standard for fitness. We were also unable to evaluate other direct parameters of the physiologic response to exercise, such as anaerobic threshold or cardiorespiratory responses, which may be abnormal in SCT carriers. Nonetheless, the modified Balke protocol is commonly used in population studies such as CARDIA, with treadmill time an accepted surrogate for fitness when direct oxygen consumption cannot be measured. The estimated METs associated with completion of the test by participants in CARDIA were, on average, in the range of vigorous intensity exercise. Finally, despite concerns of exertional rhabdomyolysis in the SCT population, biomarkers of muscle injury were not available at the time of fitness testing in CARDIA.
In summary, we report that SCT carrier status is not associated with baseline or long-term changes in cardiopulmonary fitness among a large cohort of young African American adults as they transition to middle age. In our analysis, SCT carriers did not have an increased risk of developing cardiovascular disease risk factors such as hypertension, diabetes mellitus, or metabolic syndrome during a 25-year period. Importantly, our results suggest differences in physiologic factors that may predispose SCT carriers to an increased risk of exertion-related sudden death do not affect overall fitness in this population. Further studies are warranted, however, to examine the potential differences in the physiologic response to exercise, especially high-intensity physical exertion, in individuals with SCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
CARDIA is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the NIH, National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). The CARDIA Fitness study, which collected the fitness data in 2005-2006, was funded by NHLBI grant 1 R01HL078972 (Sidney).
Authorship
Contribution: R.I.L. assisted with study design, data interpretation, and writing of the first draft of the manuscript; C.C. assisted with study design, data analysis, interpretation and manuscript editing; T.-H.T.V. assisted with data analysis, interpretation, and manuscript editing; M.F. assisted with data analysis and interpretation; A.A.T. assisted with data interpretation and manuscript editing; K.L. assisted with study design, data interpretation, and manuscript editing; and M.R.C. assisted with study design, data interpretation, and manuscript editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert I. Liem, Hematology, Oncology & Stem Cell Transplantation, Ann & Robert H. Lurie Children’s Hospital of Chicago, 225 East Chicago Ave, Box 30, Chicago, IL 60611; e-mail: rliem@luriechildrens.org.