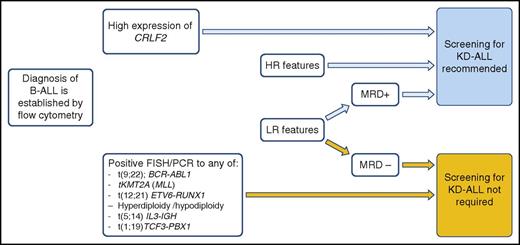
Schema for selecting B-ALL patients for KD-ALL screening. LR, low risk; MLL, mixed lineage leukemia.
Schema for selecting B-ALL patients for KD-ALL screening. LR, low risk; MLL, mixed lineage leukemia.
Screening aims at identifying HR patients and patient-specific potentially druggable targets. The proposed laboratory protocol is complex. It requires the use of multiple sophisticated methods, is costly, and time consuming. Moreover, the expected turnaround time for results is several weeks. Thus, only a limited number of specialized laboratories are up to the challenge. Nevertheless, the time has come to devise a schema for effective kinase alteration screening, which should be taken into consideration in current practice (see figure).
Patients identified by fluorescence in situ hybridization (FISH) or polymerase chain reaction tests to carry common translocations, such as BCR/ABL or others that define a specific entity according to the 2016 World Health Organization classification,5 are excluded from further screening. The highest priority for gene expression screening testing is patients presenting with HR features, those with high CRFL2 expression, and individuals with a minimal residual disease (MRD) level higher than 0.01% at the end of induction therapy. The results reported by Reshmi and colleagues support the incorporation of CRFL2 expression assessment in routine flow cytometry evaluation for B-ALL patients. In the current cohort, 80% of patients presenting with high CRFL2 expression carry a kinase-activating lesion. Both FISH and polymerase chain reaction may serve as substitutes to immunophenotyping in CRFL2 identification.
KD-ALL is more common among patients presenting with traditional HR factors. Reshmi and colleagues screened samples collected as part of clinical protocols from HR patients, patients with standard risk (SR) presenting with central nervous system or testicular involvement or those in whom MRD was detected after induction. In this subgroup of SR patients, KD-ALL was diagnosed in 17% of patients. The prevalence of KD-ALL among MRD-negative SR patients is not known.
Although the significance of this study is unequivocal, some areas of uncertainty remain. In 20% of patients presenting with high CRFL2 expression, no culprit translocation was identified. Although the false-negative rate of a low gene expression screening score is documented by the authors, the rate of false positivity is still unknown. Furthermore, a kinase-affecting alteration was not detected in 42 (14.8%) of the 284 patients defined as positive by the suggested cutoff. It is unclear whether these patients suffer from KD-ALL with their underlying activating aberration yet to be defined or if this represents false positivity of the suggested screening method. Another outstanding question is the applicability of allogeneic stem cell transplantation (allo-SCT) in KD-ALL patients achieving MRD negativity. There are scant and conflicting data available on the actual risk of relapse in MRD negative KD-ALL patients, and the outcome data of such cases following allo-SCT are likewise limited. The risk may be different for patients with ABL or CRFL2 translocation and could also be age-dependent. At least in adults, allo-SCT may be justified based on the poor general outcome reported.
Finally, it is tempting to match any found alteration with a drug that blocks the activated kinase. However, it should be emphasized that although KD-ALL patients are known to have a poor prognosis, the beneficial effect of ABL- or JAK-targeted therapies is currently based on anecdotal reports. The CRLF2/JAK aberration may be unstable in relapse6 and therefore may not be effective in such cases. The clinical experience associated with the use of TKIs in ABL-activated cases seems promising.
Ongoing prospective pediatric studies could provide evidence regarding the targeted approach in KD-ALL. As long as a clear match between a kinase-activating genetic alteration and a specific drug is not established, one should also consider the use of some of the newly approved anti-ALL antibodies as therapeutic augmentation in these HR patients.
The study by Reshmi et al is a call for action. Screening patients for KD-ALL is to be encouraged, and prospective and retrospective studies are warranted to define the optimal clinical approach in these patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal