T-cell immunotherapy against cancer, using chimeric antigen receptors (CARs) to specifically target tumor antigens without major histocompatibility complex (MHC) restriction, is widely viewed as one of the major scientific breakthroughs of recent years.1 In this issue of Blood, Yoon et al demonstrate that this approach can be adapted to redirect specificity of regulatory T cells (Tregs) to coagulation factor VIII (FVIII), thereby suppressing antibody (“inhibitor”) development in replacement therapy for hemophilia A.2
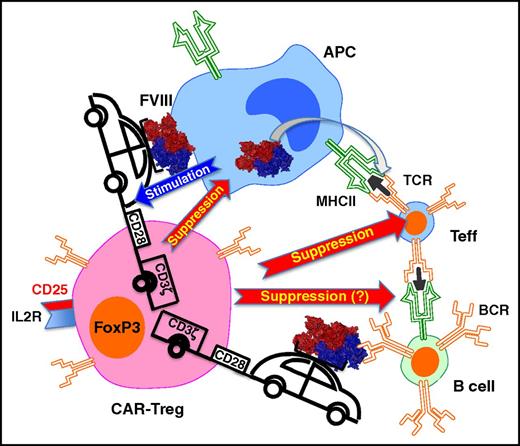
Gene-modified CD4+CD25+FoxP3+ Tregs expressing a CAR against FVIII suppress CD4+ T cells with FVIII-specific TCR and B-cell responses against FVIII. These interactions result in suppression of antibody formation against FVIII. Binding of intact FVIII protein to the CAR, possibly on the surface of an APC, stimulates the Tregs to suppress. It is possible that direct suppression of B cells with FVIII bound to their B-cell receptor (BCR) also occurs. IL2R, interleukin-2 receptor; MHC II, major histocompatibility complex II.
Gene-modified CD4+CD25+FoxP3+ Tregs expressing a CAR against FVIII suppress CD4+ T cells with FVIII-specific TCR and B-cell responses against FVIII. These interactions result in suppression of antibody formation against FVIII. Binding of intact FVIII protein to the CAR, possibly on the surface of an APC, stimulates the Tregs to suppress. It is possible that direct suppression of B cells with FVIII bound to their B-cell receptor (BCR) also occurs. IL2R, interleukin-2 receptor; MHC II, major histocompatibility complex II.
Although treatment of the X-linked bleeding disorder hemophilia is perhaps the most extensively studied example of antidrug antibody (ADA) formation against therapeutic proteins, this is a recurring problem in enzyme replacement therapies for other genetic diseases and monoclonal antibody therapies. Even though there are a number of bypass agents available to restore hemostasis in hemophilic patients with inhibitors, treatment becomes substantially more complicated, with an increased risk of morbidity and mortality. In order to resume regular FVIII replacement therapy, immune tolerance has to be established. Immune tolerance induction (ITI) is currently done through frequent high-dose FVIII infusions. These regimens and use of bypassing agents generate extraordinary costs. In addition, ITI protocols can take months or even years to complete and are not always successful.
The mammalian immune system has evolved CD4+ Tregs (characterized by constitutive expression of the transcription factor FoxP3 and of CD25, the α chain of the IL-2 receptor) that may emerge during thymic development or are peripherally induced and are essential to prevent autoimmunity. Preclinical studies have shown that such FoxP3+ Tregs also play an important role in tolerance to coagulation factor antigens used to treat hemophilia. Antigen-specific Tregs can be induced through a variety of methods, including certain types of gene therapy, transfer of tolerogenic antigen-presenting cells (APCs), coadministration of factor and immune modulatory drugs, maternal transfer, or mucosal tolerance induction.3-8 Alternatively, one could consider transplant of autologous, ex vivo expanded Tregs. FoxP3+ Tregs can be isolated from human peripheral blood by sorting for cells that are CD4+CD25+CD127lo (among other markers). Polyclonal Tregs have some efficacy but need to be given at high numbers and thus initially exert nonspecific immune suppression.5 Antigen-specific Tregs are expected to be more potent but are naturally present at very low numbers. An elegant solution to this problem is to isolate natural FoxP3+ Tregs and then redirect their specificity to FVIII by ex vivo gene transfer. Using a T-cell receptor (TCR) that had been cloned from an inhibitor patient, Scott and colleagues showed that viral vector transduced Tregs expressing the FVIII-specific TCR-suppressed proliferation of FVIII-specific CD4+ T effector (Teff) cells and anti-FVIII production by B cells.9 However, this approach would require custom TCR design depending on the patient’s HLA composition.
CAR gene transfer allows T cells to recognize a novel antigen without MHC restriction, because binding to this cell surface receptor occurs via a single-chain variable fragment (scFv), representing a fusion of the heavy and light chains of an antibody. Therefore, intact protein antigen rather than MHC–peptide complexes is bound (see figure). In this new study, an expanded collaborative team developed an FVIII-specific CAR by fusing an scFv isolated from a phage display library with a CD28 transmembrane and signaling domains and the ζ chain of the TCR complex (CD3ζ). Importantly, CAR-transduced human FoxP3+ Tregs suppressed FVIII-specific Teff and recall antibody responses in vitro with similar efficiency as TCR-transduced Tregs. Moreover, both CAR- and TCR-transduced Tregs suppressed antibody formation against FVIII in hemophilia A mice transgenic for a human HLA gene (DR1). These results provide important proof of principle that CAR-Tregs therapy is feasible to suppress ADA formation in replacement therapy for hemophilia and likely other inherited protein deficiencies.
CAR-Tregs were able to suppress responses by Teff recognizing an epitope that is in a different domain of FVIII than the B-cell epitope bound by the CAR’s scFv. Furthermore, the authors present evidence for bystander suppression. The advantage of this mode of action is that suppression, induced by stimulation with FVIII antigen, extends to all parts of the FVIII protein molecule that may contain T-cell epitopes. A disadvantage is the potential for unwanted suppression against other antigens presented by the same APC. CAR-transduced T cells should respond directly to stimulation with intact protein antigen, without a need for processing and MHC presentation by APCs. As CAR–T-cell therapy was originally developed to eliminate tumor cells expressing a specific antigen on their surface, it was however unclear whether CAR-Tregs could suppress responses to a soluble protein. Interestingly, CAR-Tregs proliferated in response to FVIII only when APCs were included, suggesting that some interaction on cell surfaces may be required. It remains to be uncovered which cell types can mediate stimulation of CAR-Tregs with antigen, and whether professional APCs are strictly required. Similarly, it is unclear how strong signal transduction has to be for optimal CAR-Tregs stimulation (including the role of ITAMs, immunoreceptor tyrosine-based activation motifs) and if inclusion of additional sequences that may help T-cell persistence and reduce cell death, such as in third-generation CARs used in CD8+ T-cell cytolytic therapy against cancer, is helpful. The in vivo effect on suppression of anti-FVIII formation in the current study was transient, possibly because of rejection or lack of survival of the human cells in the recipient mice. However, one cannot yet rule out that repeat administration of CAR-Tregs may be required for a long-term effect. However, clinical translation may be facilitated by the powerful tools established to expand human Tregs ex vivo, a tendency of T cells to persist longer after transplant in humans compared with mouse cell experiments, and the potential for infectious tolerance to additionally induce antigen-specific endogenous Tregs. Clearly, the study by Yoon and collaborators strongly supports the development of CAR-Tregs therapies to equip the immune system of hemophilic patients with the ability to eliminate the inhibitor problem. At the same time, TCR gene transfer to Tregs may still be a viable alternative because a recent study suggests that usage of FVIII-specific TCR may not be as diverse in humans as previously thought.10
Conflict-of-interest disclosure: The author declares no competing financial interests.