In this issue of Blood, Tout and colleagues add another layer of personalization to rituximab therapy in patients with diffuse large B-cell lymphoma (DLBCL) by demonstrating the relationship between baseline total metabolic tumor volume (TMTV0), rituximab exposure, and outcomes from therapy.1
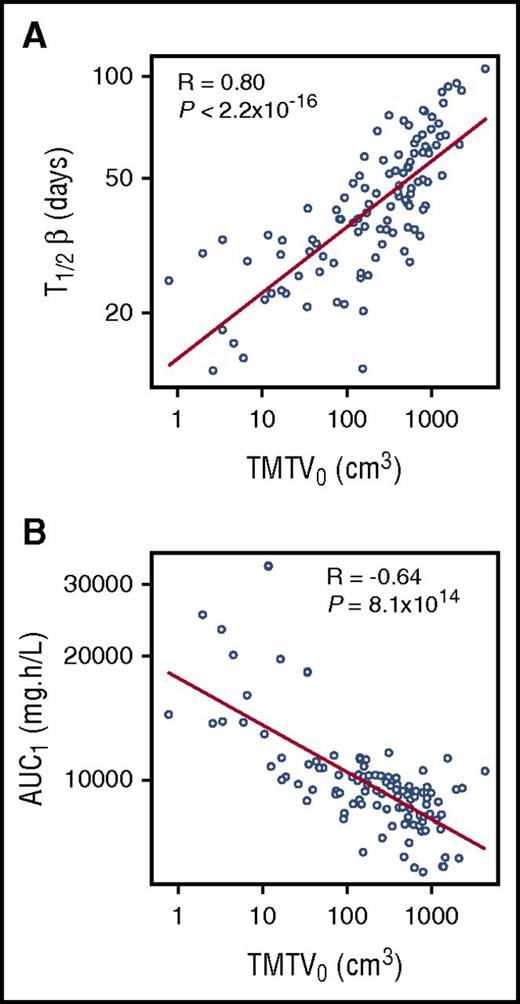
Observed relationship between rituximab pharmacokinetic parameters, (A) rituximab elimination half-life (T1/2β) and (B) area under the concentration-time curve (AUC1), relative to TMTV0. See Figure 2 in the article by Tout et al that begins on page 2616.
Observed relationship between rituximab pharmacokinetic parameters, (A) rituximab elimination half-life (T1/2β) and (B) area under the concentration-time curve (AUC1), relative to TMTV0. See Figure 2 in the article by Tout et al that begins on page 2616.
The foundation of precision medicine or personalized therapy is the idea that, for each patient, we can select the best available drug monotherapy or combination of therapies and the optimal dose regimen for this therapy to achieve maximum benefit for the patient. Rituximab was approved by the US Food and Drug Administration for use in DLBCL nearly 20 years ago. It was the first immunotherapy approved for specific use in cancer, and it quickly demonstrated a dramatic improvement in outcomes in patients with DLBCL when combined with chemotherapeutic agents.2,3 Rituximab has since been approved for use in chronic lymphocytic leukemia, follicular lymphoma, and rheumatoid arthritis. It specifically targets CD20, which is expressed on normal B lymphocytes and on several types of malignant B cells. As an immunotherapy, rituximab is already a “personalized therapeutic” because patients whose tumors express higher levels of CD20 are expected to receive greater benefit from rituximab use. Not surprisingly, increased CD20 expression has been demonstrated to be correlated with improved survival in DLBCL.4 Furthermore, others had already demonstrated that higher rituximab exposure correlates with improved outcomes from therapy.5
But, it’s not that simple, of course.
CD20 expression also influences the disposition of rituximab. This phenomenon is referred to as target-mediated drug disposition (TMDD), and it can occur when a substantial portion of a drug binds with high affinity and high specificity to a target.6 In cases where the drug binds its target irreversibly or is internalized and degraded after binding to its target, this binding can contribute directly to drug clearance. In other cases where the drug binds tightly but reversibly, receptor binding may serve as a distribution compartment, thus impacting the drug’s overall volume of distribution. TMDD can occur with small molecules,7 but it is more commonly observed with antibody therapeutics, including rituximab.8
In the current study by Tout and colleagues, 18F-fluorodeoxyglucose-positron emission tomography (PET)–computed tomography was used to measure TMTV0 in 108 patients that were also assessed for rituximab pharmacokinetics. Because higher TMTV0 (ie, higher tumor burden at baseline) would be expected to correlate with higher total CD20 expression, the investigators evaluated the influence of TMTV0 on rituximab pharmacokinetics. Not surprisingly, they observed a strong correlation between TMTV0 and rituximab disposition as demonstrated in the figure. Notably, although other groups have incorporated time-varying clearance to account for the target-mediated behavior of rituximab,5,8 Tout and colleagues have attributed this behavior to changes in the volume of distribution. In either case, the higher the quantity of receptor present, the greater the influence on drug disposition.
In addition to progression-free and overall survival, PET imaging after 4 cycles of either R-CHOP-14 (rituximab, doxorubicin, cyclophosphamide, vincristine, and prednisone) or R-ACVBP-14 (rituximab, doxorubicin, cyclophosphamide, vincristine, bleomycin, and prednisone) was also available to provide a binary assessment of the complete metabolic response to treatment in 97 of the 108 patients. Again, as expected, the investigators observed relationships between rituximab pharmacokinetics and outcomes (progression-free survival, overall survival, and metabolic response, as shown in Figure 2C-F of Tout et al). A separate group recently reported metabolic tumor volume was associated with outcomes in DLBCL patients with bone marrow involvement.9 The additional contribution made in this current report by Tout and colleagues is that high TMTV0, which correlates with less favorable outcomes in patients receiving the standard 375 mg/m2 dose of rituximab, also correlates with relatively low rituximab exposures. This same group had previously linked rituximab pharmacokinetics, tumor burden, and outcomes in a mouse model of lymphoma,10 but the current report is the first to provide a clinical data set demonstrating the link between all 3 of these variables.
Importantly, standard dosing in DLBCL with rituximab has remained at 375 mg/m2 since early in its clinical development. Tout and colleagues have also constructed a nomogram based on their data, which provides a rational scheme for increasing the rituximab dose in patients with high TMTV0 to achieve rituximab exposures that have a better chance of prolonging the duration of response. Thus, this work provides strong rationale and even a path forward for considering personalized rituximab dosing based on individual TMTV0 in patients with DLBCL.
There are many other immunotherapies that have been approved or are in development, and these target CD20 plus a variety of other antigens. Given that the basic behavior of these therapies will be similar to rituximab (ie, high-affinity and high-specificity binding to a single cancer-associated antigen), we can expect that similar relationships between target expression, pharmacokinetic exposure of these antibodies, and outcomes from therapy may exist, thus indicating that personalized dosing strategies may be feasible in diseases where target expression and/or tumor burden can be quantified. A recent review by Ku, Chong, and Hawkes3 nicely summarizes immunotherapies more recently approved or in development for B-cell malignancies, and they also point out that dosing regimens could be more optimally tuned for individuals. Although the proposed dosing nomogram for rituximab presented by Tout and colleagues must be further tested and validated, future personalized dosing regimen designs for other existing and new antibody therapies could benefit from the lessons learned with rituximab and the approaches taken by this group.
Conflict-of-interest disclosure: The author declares no competing financial interests.