Key Points
Eliglustat treatment resulted in stable mean hemoglobin concentration, platelet count, and spleen and liver volumes for up to 4 years.
Mean bone mineral density z scores also remained stable and were maintained in the healthy reference range for up to 4 years.
Abstract
In the phase 3 Study of Eliglustat Tartrate (Genz-112638) in Patients With Gaucher Disease Who Have Reached Therapeutic Goals With Enzyme Replacement Therapy (ENCORE), at 1 year, eliglustat was noninferior to imiglucerase enzyme therapy in maintaining stable platelet counts, hemoglobin concentrations, and spleen and liver volumes. After this primary analysis period, patients entered a long-term extension phase in which all received eliglustat. Duration on eliglustat ranged from 2 to 5 years, depending on timing of enrollment (which spanned 2 years), treatment group to which patients were randomized, and whether they lived in the United States when commercial eliglustat became available. Here we report long-term safety and efficacy of eliglustat for 157 patients who received eliglustat in the ENCORE trial; data are available for 46 patients who received eliglustat for 4 years. Mean hemoglobin concentration, platelet count, and spleen and liver volumes remained stable for up to 4 years. Year to year, all 4 measures remained collectively stable (composite end point relative to baseline values) in ≥85% of patients as well as individually in ≥92%. Mean bone mineral density z scores (lumbar spine and femur) remained stable and were maintained in the healthy reference range throughout. Eliglustat was well tolerated over 4 years; 4 (2.5%) patients withdrew because of adverse events that were considered related to the study drug. No new or long-term safety concerns were identified. Clinical stability assessed by composite and individual measures was maintained in adults with Gaucher disease type 1 treated with eliglustat who remained in the ENCORE trial for up to 4 years. This trial was registered at www.clinicaltrials.gov as #NCT00943111.
Introduction
Gaucher disease type 1 is an inherited lysosomal disorder characterized by deficient activity of the enzyme acid β-glucosidase with consequential accumulation of the substrate, glucosylceramide, and its unacylated derivative, glucosylsphingosine, primarily in lysosomes of tissue macrophages.1 Pathological accumulation of these glycosphingolipids is associated with multisystemic disease manifestations, most notably hepatosplenomegaly, accompanied by anemia, thrombocytopenia, and bone disease.1
For 25 years, enzyme replacement therapy for Gaucher disease type 1 has been the mainstay of treatment. This usually involves alternate-week infusions of recombinant mannose-terminated human acid β-glucosidase, which is targeted to the pathological macrophages, where it augments the residual enzyme activity to enhance recycling of β-glucosylceramide. Enzyme therapy can reverse the hematological and visceral complications of the disease and can prevent bone damage; it improves the quality of life for people with Gaucher disease.2-5 Eliglustat is an oral substrate reduction therapy approved in the United States in 2014 and the European Union in 2015 for adults with Gaucher disease type 1 who are extensive, intermediate, or poor CYP2D6 metabolizers (>90% of patients).6,7 Eliglustat acts by partially inhibiting the de novo biosynthesis of β-glucosylceramide, thereby rebalancing the rate of formation of the primary substrate of the deficient enzyme with its impaired degradation. In clinical phase 2 and 3 studies of previously untreated patients with Gaucher disease type 1, eliglustat induced clinically meaningful improvements in hematological parameters as well as spleen and liver volumes at 9 to 12 months,8,9 which were maintained at 18 months10 and after 4 years.11 Bone mineralization density also continued to improve after 1 to 4 years of eliglustat.11-13
In the phase 3 Study of Eliglustat Tartrate (Genz-112638) in Patients With Gaucher Disease Who Have Reached Therapeutic Goals With Enzyme Replacement Therapy (ENCORE), after 1 year of treatment, eliglustat was found to be noninferior to imiglucerase in maintaining stable platelet and hemoglobin parameters as well as spleen and liver volumes.14 Bone parameters and quality-of-life measures also remained stable.14 After the 12-month primary analysis period, patients were offered enrollment in a long-term extension phase during which all received eliglustat. Here we report the safety and efficacy outcomes from the ENCORE trial over the entire trial.
Methods
Study design
The ENCORE clinical trial was a randomized, multinational, open-label, noninferiority study comparing eliglustat (Cerdelga; Sanofi Genzyme, Cambridge, MA) with imiglucerase (Cerezyme; Sanofi Genzyme) as a maintenance therapy in patients with Gaucher disease type 1 who had already achieved therapeutic goals while receiving enzyme therapy. Detailed methods and the primary outcomes from ENCORE were published previously.14 All long-term efficacy and safety analyses were done on the intent-to-treat population. As in the primary analysis, dosing was individualized and based on achieving plasma trough levels of at least 5 ng/mL (with doses of 50, 100, or 150 mg eliglustat tartrate twice daily); because the Cerdelga product label refers to active base, this corresponds to doses of 42, 84, and 127 mg, respectively. After the 12-month primary analysis period, all patients had the option of continuing in the open-label extension phase of the trial, in which they were treated with eliglustat (ie, patients initially randomized to imiglucerase were switched to eliglustat) and followed until the predetermined end of the study in May 2015. Thus, patients had the opportunity to be treated with eliglustat for 2 to 4.5 years. This was determined by when they enrolled (enrollment spanned 2 years: from September 2009 until November 2011), the initial treatment group to which they were randomly assigned, and their country of residence. After approval of the drug by the US Food and Drug Administration in fall 2014, trial participants living in the United States were discontinued from the study and transitioned to commercial eliglustat.
In the analysis of long-term treatment with eliglustat, baseline was defined as the last available assessment before eliglustat treatment initiation (day 1 for patients originally randomized to eliglustat and week 52 + 1 day for patients originally randomized to imiglucerase). All data were analyzed with respect to time on eliglustat treatment rather than time in the trial. Patients were not distinguished on the basis of their original treatment allocation because all patients, including those randomized to receive imiglucerase, were receiving enzyme therapy before switching to eliglustat.
Here we report long-term safety and efficacy with respect to years of exposure for all 157 eliglustat-treated patients in ENCORE; in 46 of these, data are available for a period of 4 years.
Assessments
The following parameters were evaluated, as described previously: hemoglobin concentration, platelet count, spleen volume, liver volume, bone mineral density, biomarkers reflecting the activity of Gaucher disease (plasma chitotriosidase activity [normalized, nanomoles per hour per milliliter], plasma glucosylceramide [GL-1, micrograms per milliliter], ganglioside GM3 [micrograms per milliliter], macrophage inflammatory protein 1β [picograms per milliliter], ceramide [milligrams per liter], and sphingomyelin [micrograms per milliliter]), and quality-of-life measures (mobility, bone pain, Fatigue Severity Score, 36-Item Short Form Survey, Brief Pain Inventory, and Gaucher Disease Severity Score).14 For these analyses, values were examined at baseline before eliglustat was given, and after 1, 2, 3, and 4 years of treatment with the agent. Adverse events (AEs) were summarized by their incidence, seriousness, severity, and relationship to eliglustat and to underlying disease.
Analysis of disease stability
As described previously, the composite primary efficacy end point of the ENCORE trial was the percentage of patients whose hematological parameters and organ volumes remained stable after 12 months.14 The stability criteria were defined as those established for these measures in patients with Gaucher disease type 1 receiving maintenance treatment with imiglucerase15 : hemoglobin concentration that did not decrease by more than 1.5 g/dL; platelet count that did not decrease by more than 25%; spleen volume (in nonsplenectomized subjects expressed as multiples of normal [MNs]) that did not increase by more than 25%; and liver volume (MN) that did not increase by more than 20% from baseline.
We also determined whether patients met the following prespecified therapeutic goals for hemoglobin, platelet, spleen, and liver parameters based on trial entry criteria and established therapeutic goals for patients receiving enzyme therapy15-17 : hemoglobin ≥11.0 g/dL for women and ≥12.0 g/dL for men; platelet count ≥100 × 109/L; spleen volume ≤8 MN; and liver volume ≤1.5 MN.
Statistical analysis
The analyses include all patients who were treated with eliglustat and were carried out with respect to the time (in years) of exposure to eliglustat and not time of engagement in the trial. For hemoglobin, platelets, spleen volume, liver volume, lumbar spine z score, and femur z score, means, and 95% confidence intervals over time were determined for the full trial population and for the subset of patients who had 4-year data; these are presented graphically for the baseline, year 1, year 2, year 3, and year 4 trial visits. A repeated-measures mixed model was undertaken for hemoglobin concentration, platelet count, spleen volume, liver volume, and lumbar spine and femur z scores to assess linear trends during the prolonged course of this trial. Predictor variables included the corresponding baseline assessment and time on eliglustat. A logistic regression analysis was done to ascertain if failure to achieve the primary composite endpoint at any time in the trial correlated with age (as a continuous variable), splenectomy status (spleen/no spleen), or obesity status (obese/nonobese).
Achievement of stability with respect to the trial end points (individually for hemoglobin, platelets, spleen volume, and liver volume as well as the composite primary end point of all 4 stability parameters) has been summarized by parameter and by year of visit. Stability with respect to the predefined absolute therapeutic goal thresholds for hemoglobin, platelets, spleen volume, and liver volume is also summarized by parameter and year, individually and collectively. For both measures of stability, the percentages of patients reaching the goals and binomial exact 95% confidence intervals were calculated based on the number of patients at risk (ie, patients with a measurement for the parameter of interest) at each visit for each goal individually and for all 4 goals collectively.
AEs have been set out in terms of relatedness to eliglustat treatment. In particular, events deemed related by investigators that occurred in ≥5% of patients have been presented by number of patients as well as the number of events. Percentages of patients in each category were calculated from the total number treated with eliglustat in the 1-year primary analysis study or during the extension period. Compliance was determined by the number of pills that were returned at each study visit.
Results
Patient disposition and characteristics
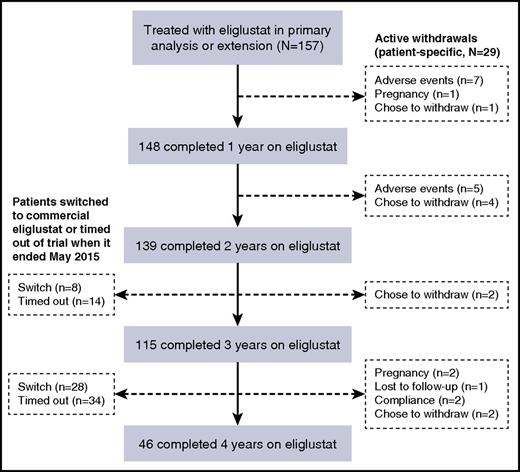
Of 157 patients who entered the trial and were treated with eliglustat, 148 completed 1 year of treatment with eliglustat, 139 completed 2 years, 115 completed 3 years, and 46 completed 4 years of treatment (Figure 1). Of the 111 patients who did not complete 4 years of treatment with eliglustat, 36 switched to commercial eliglustat when it became available in fall 2014 and 48 patients timed out of the trial for logistical reasons when it ended before they had accrued 4 years of follow-up. Overall, 12 patients withdrew from the trial because of an AE, 10 decided to withdraw for reasons unrelated to AEs (including 1 withdrawal after 4 years of treatment), 4 because of pregnancy (1 pregnancy occurred after 4 years of treatment), 2 on account of noncompliance, and 1 subject was lost to follow-up as a result of international travel. A further 15 patients moved to commercial eliglustat after receiving eliglustat for 4 years but before the trial had ended. Demographic and baseline disease characteristics for the eliglustat-treated patients who completed 4 years of treatment were similar to those for all eliglustat-treated patients who entered the trial (Table 1).
Demographics and baseline patient characteristics
| Parameter . | All eliglustat-treated patients (N = 157) . | Extension patients with 4-y data (N = 46) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 72 (46) | 20 (44) |
| Female | 85 (54) | 26 (57) |
| Age at start of eliglustat, mean ± SD, y* | 38.0 ± 14.0 | 34.0 ± 14.4 |
| Age at first symptom, mean ± SD, y | 13.9 ± 12.8 | 10.1 ± 8.6 |
| Age at diagnosis, mean ± SD, y | 18.8 ± 13.9 | 14.8 ± 11.9 |
| Gaucher disease genotype, n (%) | ||
| L444P/other | 2 (1.3) | 0 |
| N370S/L444P | 56 (35.7) | 21 (45.7) |
| N370S/N370S | 35 (22.3) | 6 (13.0) |
| N370S/other | 47 (29.9) | 17 (37.0) |
| Other/other | 17 (10.8) | 2 (4.3) |
| Splenectomy performed, n (%) | ||
| Partial | 2 (1) | 2 (4) |
| Total | 37 (24) | 13 (28) |
| Years on enzyme therapy before eliglustat, mean ± SD | 10.5 ± 4.1 | 9.4 ± 4.5 |
| CYPD2D6 metabolizer status, n (%) | ||
| Poor | 6 (3.8) | 3 (6.5) |
| Intermediate | 21 (13.4) | 3 (6.5) |
| Extensive | 122 (77.7) | 37 (80.4) |
| Ultrarapid | 5 (3.2) | 3 (6.5) |
| Indeterminate | 3 (1.9) | 0 |
| Parameter . | All eliglustat-treated patients (N = 157) . | Extension patients with 4-y data (N = 46) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 72 (46) | 20 (44) |
| Female | 85 (54) | 26 (57) |
| Age at start of eliglustat, mean ± SD, y* | 38.0 ± 14.0 | 34.0 ± 14.4 |
| Age at first symptom, mean ± SD, y | 13.9 ± 12.8 | 10.1 ± 8.6 |
| Age at diagnosis, mean ± SD, y | 18.8 ± 13.9 | 14.8 ± 11.9 |
| Gaucher disease genotype, n (%) | ||
| L444P/other | 2 (1.3) | 0 |
| N370S/L444P | 56 (35.7) | 21 (45.7) |
| N370S/N370S | 35 (22.3) | 6 (13.0) |
| N370S/other | 47 (29.9) | 17 (37.0) |
| Other/other | 17 (10.8) | 2 (4.3) |
| Splenectomy performed, n (%) | ||
| Partial | 2 (1) | 2 (4) |
| Total | 37 (24) | 13 (28) |
| Years on enzyme therapy before eliglustat, mean ± SD | 10.5 ± 4.1 | 9.4 ± 4.5 |
| CYPD2D6 metabolizer status, n (%) | ||
| Poor | 6 (3.8) | 3 (6.5) |
| Intermediate | 21 (13.4) | 3 (6.5) |
| Extensive | 122 (77.7) | 37 (80.4) |
| Ultrarapid | 5 (3.2) | 3 (6.5) |
| Indeterminate | 3 (1.9) | 0 |
SD, standard deviation.
Age on day 1 of first dose of eliglustat.
Maintenance of stable Gaucher disease hematological and visceral parameters
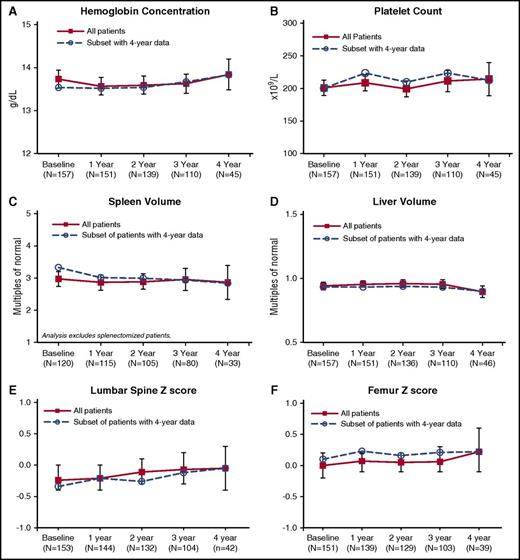
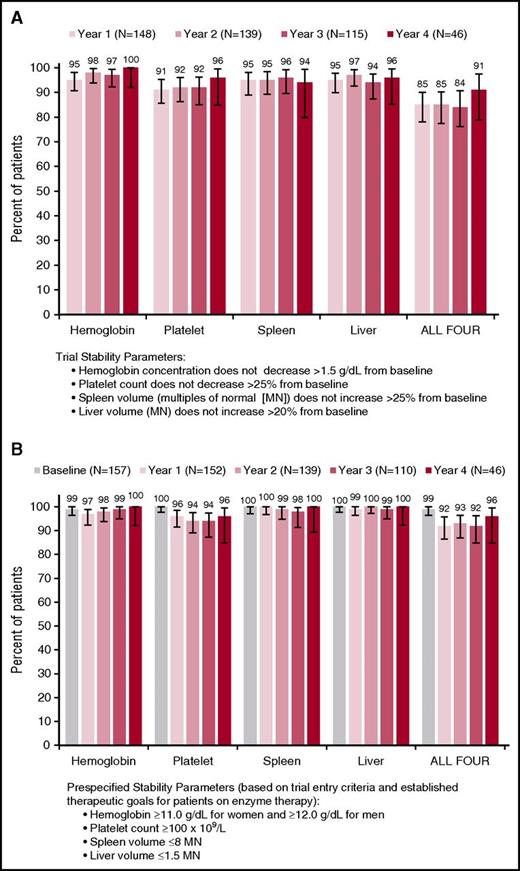
Throughout the trial, mean values for hemoglobin concentration, platelet count, spleen volume, and liver volume remained stable among all eliglustat-treated patients and in the subset of patients who had 4-year data (Figure 2A-D), with no clinically significant changes from the baseline values attained after a mean of 10 years of enzyme therapy. There were small but statistically significant reductions in least-square mean liver (3%, P = .03) and spleen volumes (13%, P < .0001) after 4 years of eliglustat treatment (Table 2). With respect to the primary and secondary clinical end points, year to year, all 4 measures remained collectively stable (composite primary end point relative to baseline values) in ≥85% of patients and individually in ≥92% of patients (secondary end points) (Figure 3A). With respect to prespecified therapeutic goals established for patients on enzyme therapy,16,17 thresholds for hemoglobin, platelets, spleen, and liver were maintained collectively in ≥92% of patients and each individual goal was maintained in ≥94% of patients (Figure 3B).
Mean for hematologic, visceral, and bone parameters over 4 years of eliglustat treatment. Error bars denote upper and lower 95% confidence intervals.
Mean for hematologic, visceral, and bone parameters over 4 years of eliglustat treatment. Error bars denote upper and lower 95% confidence intervals.
LS mean changes from baseline to 4 y in hematological, visceral, and bone parameters from repeated-measures mixed model
| Parameter . | LS mean (95% CI) . | LS mean change from baseline (95% CI) . | P value . |
|---|---|---|---|
| Hemoglobin, g/dL | |||
| B (n = 157) | 13.7 (13.6, 13.8) | — | — |
| Year 1 (n = 151) | 13.6 (13.4, 13.7) | −0.11 (−0.27, 0.06) | .21 |
| Year 2 (n = 139) | 13.6 (13.4, 13.7) | −0.09 (−0.27, 0.09) | .31 |
| Year 3 (n = 110) | 13.6 (13.5, 13.8) | −0.04 (−0.23, 0.15) | .67 |
| Year 4 (n = 45) | 13.9 (13.7, 14.1) | 0.23 (−0.02, 0.49) | .07 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .06 |
| Platelet count, ×109/L | |||
| B (n = 157) | 200.3 (194.4, 206.1) | — | — |
| Year 1 (n = 151) | 206.7 (200.7, 212.7) | 6.31 (−1.52, 14.13) | .11 |
| Year 2 (n = 139) | 202.4 (196.2, 208.6) | 2.13 (−6.35, 10.60) | .62 |
| Year 3 (n = 110) | 210.7 (203.8, 217.6) | 10.40 (1.35, 19.45) | .02 |
| Year 4 (n = 45) | 209.9 (199.2, 220.7) | 9.57 (−2.60, 21.74) | .12 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .09 |
| Spleen volume, MN | |||
| B (n = 120) | 3.1 (3.0, 3.2) | — | — |
| Year 1 (n = 115) | 2.9 (2.9, 3.0) | −0.14 (−0.22, −0.05) | .002 |
| Year 2 (n = 105) | 2.9 (2.8, 3.0) | −0.20 (−0.31, −0.09) | .0003 |
| Year 3 (n = 80) | 2.9 (2.8, 3.0) | −0.21 (−0.35, −0.10) | .0003 |
| Year 4 (n = 33) | 2.7 (2.5, 2.8) | −0.39 (−0.55, −0.22) | <.0001 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | <.0001 |
| Liver volume, MN | |||
| B (n = 157) | 0.94 (0.93, 0.96) | — | — |
| Year 1 (n = 151) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .17 |
| Year 2 (n = 136) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .29 |
| Year 3 (n = 110) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .49 |
| Year 4 (n = 46) | 0.91 (0.89, 0.94) | −0.03 (−0.06, −0.004) | .03 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .04 |
| Total spine z score | |||
| B (n = 153) | −0.3 (−0.3, −0.2) | — | — |
| Year 1 (n = 144) | −0.2 (−0.2, −0.1) | 0.07 (0.03, 0.11) | .002 |
| Year 2 (n = 132) | −0.1 (−0.2, −0.09) | 0.11 (0.06, 0.17) | <.0001 |
| Year 3 (n = 104) | −0.09 (−0.1, −0.04) | 0.16 (0.10, 0.23) | <.0001 |
| Year 4 (n = 42) | 0.04 (−0.04, 0.1) | 0.29 (0.20, 0.38) | <.0001 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | <.0001 |
| Total femur z score | |||
| B (n = 151) | 0.03 (−0.007, 0.060) | — | — |
| Year 1 (n = 139) | 0.07 (0.034, 0.103) | 0.04 (0.013, 0.072) | .005 |
| Year 2 (n = 129) | 0.05 (0.018, 0.088) | 0.03 (−0.012, 0.065) | .17 |
| Year 3 (n = 103) | 0.08 (0.044, 0.120) | 0.06 (0.011, 0.101) | .02 |
| Year 4 (n = 39) | 0.07 (0.018, 0.131) | 0.05 (−0.015, 0.111) | .13 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .14 |
| Parameter . | LS mean (95% CI) . | LS mean change from baseline (95% CI) . | P value . |
|---|---|---|---|
| Hemoglobin, g/dL | |||
| B (n = 157) | 13.7 (13.6, 13.8) | — | — |
| Year 1 (n = 151) | 13.6 (13.4, 13.7) | −0.11 (−0.27, 0.06) | .21 |
| Year 2 (n = 139) | 13.6 (13.4, 13.7) | −0.09 (−0.27, 0.09) | .31 |
| Year 3 (n = 110) | 13.6 (13.5, 13.8) | −0.04 (−0.23, 0.15) | .67 |
| Year 4 (n = 45) | 13.9 (13.7, 14.1) | 0.23 (−0.02, 0.49) | .07 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .06 |
| Platelet count, ×109/L | |||
| B (n = 157) | 200.3 (194.4, 206.1) | — | — |
| Year 1 (n = 151) | 206.7 (200.7, 212.7) | 6.31 (−1.52, 14.13) | .11 |
| Year 2 (n = 139) | 202.4 (196.2, 208.6) | 2.13 (−6.35, 10.60) | .62 |
| Year 3 (n = 110) | 210.7 (203.8, 217.6) | 10.40 (1.35, 19.45) | .02 |
| Year 4 (n = 45) | 209.9 (199.2, 220.7) | 9.57 (−2.60, 21.74) | .12 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .09 |
| Spleen volume, MN | |||
| B (n = 120) | 3.1 (3.0, 3.2) | — | — |
| Year 1 (n = 115) | 2.9 (2.9, 3.0) | −0.14 (−0.22, −0.05) | .002 |
| Year 2 (n = 105) | 2.9 (2.8, 3.0) | −0.20 (−0.31, −0.09) | .0003 |
| Year 3 (n = 80) | 2.9 (2.8, 3.0) | −0.21 (−0.35, −0.10) | .0003 |
| Year 4 (n = 33) | 2.7 (2.5, 2.8) | −0.39 (−0.55, −0.22) | <.0001 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | <.0001 |
| Liver volume, MN | |||
| B (n = 157) | 0.94 (0.93, 0.96) | — | — |
| Year 1 (n = 151) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .17 |
| Year 2 (n = 136) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .29 |
| Year 3 (n = 110) | 0.95 (0.94, 0.97) | 0.01 (−0.01, 0.03) | .49 |
| Year 4 (n = 46) | 0.91 (0.89, 0.94) | −0.03 (−0.06, −0.004) | .03 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .04 |
| Total spine z score | |||
| B (n = 153) | −0.3 (−0.3, −0.2) | — | — |
| Year 1 (n = 144) | −0.2 (−0.2, −0.1) | 0.07 (0.03, 0.11) | .002 |
| Year 2 (n = 132) | −0.1 (−0.2, −0.09) | 0.11 (0.06, 0.17) | <.0001 |
| Year 3 (n = 104) | −0.09 (−0.1, −0.04) | 0.16 (0.10, 0.23) | <.0001 |
| Year 4 (n = 42) | 0.04 (−0.04, 0.1) | 0.29 (0.20, 0.38) | <.0001 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | <.0001 |
| Total femur z score | |||
| B (n = 151) | 0.03 (−0.007, 0.060) | — | — |
| Year 1 (n = 139) | 0.07 (0.034, 0.103) | 0.04 (0.013, 0.072) | .005 |
| Year 2 (n = 129) | 0.05 (0.018, 0.088) | 0.03 (−0.012, 0.065) | .17 |
| Year 3 (n = 103) | 0.08 (0.044, 0.120) | 0.06 (0.011, 0.101) | .02 |
| Year 4 (n = 39) | 0.07 (0.018, 0.131) | 0.05 (−0.015, 0.111) | .13 |
| Test for linear trend (B, 1, 2, 3, 4 y) | — | — | .14 |
B, baseline; CI, confidence interval.
Stability of hematologic and visceral parameters. (A) Composite primary end point (relative to change from baseline) and (B) prespecified therapeutic goals based on entry criteria and goals established for patients on enzyme therapy (absolute value). Error bars denote upper and lower 95% confidence intervals.
Stability of hematologic and visceral parameters. (A) Composite primary end point (relative to change from baseline) and (B) prespecified therapeutic goals based on entry criteria and goals established for patients on enzyme therapy (absolute value). Error bars denote upper and lower 95% confidence intervals.
Most patients who did not achieve the primary composite end point missed on a single criterion, and this group varied from year to year. Only 1 patient missed more than 2 criteria at once; this patient missed spleen, platelets, and liver at 1 time point. Among patients with at least 2 years on eliglustat, 6 missed the composite end point at all time points (5 consistently missed the platelet end point, 3 missed the spleen end point 1 or more times, and 1 missed the liver end point 1 time). No common clinical characteristics in this group were identified. The logistic regression analysis found no correlation between age, sex, splenectomy status, or obesity status with inability to meet the composite end point.
Maintenance of stable bone mineral density
Active bone disease was an exclusion criterion for this trial; patients could not have experienced bone crisis or symptomatic bone disease (bone pain attributable to osteonecrosis or pathological fractures within the year before randomization).14 Mean baseline bone mineral density scores for the lumbar spine and femur were found to be within the age-matched reference range for healthy individuals. These values were maintained throughout the 4 years of eliglustat treatment (Figure 2E-F). Of note, after 4 years of treatment with eliglustat, lumbar spine least-square mean z scores increased by 0.29 (P < .0001) (Table 2).
Biomarkers, Gaucher disease severity, and quality of life
The respective activity measures and concentrations of disease-related biomarkers are set out in Table 3. Median activities of chitotriosidase and concentrations of macrophage inflammatory protein 1β were elevated at baseline and decreased modestly over 4 years. Plasma concentrations of GL-1 and GM3 ganglioside were in the healthy reference range at baseline but decreased by >50% during the first 3 months of exposure to eliglustat, consistent with the systemic action and diffusible nature of this agent; thereafter, these biomarkers remained stable and within the healthy normal range during the investigation period up to 4 years. Median concentrations of the bioactive sphingolipids, ceramide and sphingomyelin, were in the normal reference range at baseline; over 4 years, ceramide was unchanged and sphingomyelin increased slightly but remained well within the normal reference range for healthy subjects.
Median changes in plasma biomarkers from baseline to 4 y
| End point . | Baseline median (min, max) . | 4-y median (min, max) . | Change from baseline median (min, max) . | Percent change from baseline median (min, max) . |
|---|---|---|---|---|
| Normalized chitotriosidase activity, nmol/h/mL | 605 (0, 10 761); n = 99 | 363 (4, 3916); n = 30 | −310 (−3397, 859); n = 26 | −63 (−98, 81); n = 26 |
| Plasma GL-1, μg/mL | 5.10 (2.2, 16.9); n = 155 | 2.00 (2.0, 5.3); n = 42 | −3.35 (−8.4, 0.4); n = 42 | −60.36 (−80.8, 8.2); n = 42 |
| Plasma GM3, μg/mL | 13.0 (7, 30); n = 136 | 6.0 (4, 15); n = 43 | −7.0 (−23, 0); n = 29 | −55.6 (−82, 0); n = 29 |
| Plasma ceramide, mg/L | 3.90 (2.2, 8.3); n = 154 | 3.80 (2.5, 6.4); n = 43 | −0.60 (−2.4, 1.6); n = 43 | −13.33 (−40.0, 66.7); n = 43 |
| Plasma sphingomyelin, μg/mL | 318.0 (200, 596); n = 154 | 434.0 (224, 645); n = 43 | 133.0 (0, 362); n = 43 | 41.8 (0, 127); n = 43 |
| Plasma macrophage inflammatory protein 1β, pg/mL | 51.65 (9.3, 433.8); n = 152 | 55.30 (15.6, 193.7); n = 38 | −21.78 (−279.3, 114.4); n = 38 | −31.45 (−83.3, 289.3); n = 38 |
| End point . | Baseline median (min, max) . | 4-y median (min, max) . | Change from baseline median (min, max) . | Percent change from baseline median (min, max) . |
|---|---|---|---|---|
| Normalized chitotriosidase activity, nmol/h/mL | 605 (0, 10 761); n = 99 | 363 (4, 3916); n = 30 | −310 (−3397, 859); n = 26 | −63 (−98, 81); n = 26 |
| Plasma GL-1, μg/mL | 5.10 (2.2, 16.9); n = 155 | 2.00 (2.0, 5.3); n = 42 | −3.35 (−8.4, 0.4); n = 42 | −60.36 (−80.8, 8.2); n = 42 |
| Plasma GM3, μg/mL | 13.0 (7, 30); n = 136 | 6.0 (4, 15); n = 43 | −7.0 (−23, 0); n = 29 | −55.6 (−82, 0); n = 29 |
| Plasma ceramide, mg/L | 3.90 (2.2, 8.3); n = 154 | 3.80 (2.5, 6.4); n = 43 | −0.60 (−2.4, 1.6); n = 43 | −13.33 (−40.0, 66.7); n = 43 |
| Plasma sphingomyelin, μg/mL | 318.0 (200, 596); n = 154 | 434.0 (224, 645); n = 43 | 133.0 (0, 362); n = 43 | 41.8 (0, 127); n = 43 |
| Plasma macrophage inflammatory protein 1β, pg/mL | 51.65 (9.3, 433.8); n = 152 | 55.30 (15.6, 193.7); n = 38 | −21.78 (−279.3, 114.4); n = 38 | −31.45 (−83.3, 289.3); n = 38 |
Normalized chitotriosidase: values were doubled for patients who had heterozygous chitotriosidase genotypes for the common null (24 bp duplication) mutation. Patients who have a chitotriosidase genotyping category of “homozygous mutation” have no expected chitotriosidase activity; therefore, their values for below the level of quantification were set equal to missing for this analysis. Normal ranges: chitotriosidase 4-120 nmol/h/mL; plasma GL-1, ≤2.0-6.6 μg/mL; GM3, 5-21 μg/mL; ceramide, 1.8-6.5 μg/mL; sphingomyelin, <200-703 μg/mL; macrophage inflammatory protein 1β, 27.3-77.2 pg/mL.
Max, maximum; min, minimum.
No clinically significant changes in any Gaucher disease or quality-of-life measures were observed over 4 years. The mean baseline Fatigue Severity Scale score was 3.0 (1 = least severe, 7 = most severe) and remained between 3.0 and 3.2 (median scores, 2.7-2.9). The mean baseline values for the 36-Item Short Form Survey mental and physical component scores were 52 and 51, respectively (mean score for a normal comparator population = 50); both remained between 50 and 51 (median scores, 52-56). For the Brief Pain Inventory, mean average pain score at baseline was 1.4 (0 = noninterference, 10 = maximal interference) and remained between 1.4 and 1.6 (median score, 0-1). For the Gaucher Disease Severity Scoring System, the mean total baseline score was 2.2 (0 = best, 19 = worst score) and remained between 2.0 to 2.3 (median, 2.0 at all time points). At baseline, 95% of patients reported unrestricted mobility; this percentage remained between 95% and 97% year to year. Three patients (2%) reported a bone crisis while being treated with eliglustat, including 1 who also had a bone crisis retrospectively identified at baseline. Of the 141 patients who responded to a treatment preference survey (oral vs IV enzyme treatment) after 1 year of eliglustat, 98% expressed a preference for oral therapy; the main reason cited was convenience. Of the 3 patients who did not prefer oral treatment, 2 were undecided and 1 preferred IV treatment because it was given in the hospital.
Compliance
Overall, 90% of patients took ≥90% of their pills, 6% took ≥80% to <90% of their pills, 3% took ≥70% to <80%, and 2% took ≥40% to <60% of their pills.
AEs
The AE data set out in Table 4 reflect 511 patient-years of exposure to eliglustat. Seventy-four percent of AEs were mild, 23% were moderate, and 3% were considered severe. Four (2.5%) patients withdrew because of AEs considered related to eliglustat, all of which occurred during the patients’ first 9 months of eliglustat treatment and all resolved: mild lethargy and exfoliative rash (0.1 years), severe upper abdominal pain (0.3 years), moderate palpitations (0.54 years) with no clinically relevant electrocardiographic findings, and mild vertigo (0.83 years). Two serious AEs considered possibly related to eliglustat by the investigator occurred in 2 patients; neither led to withdrawal from the study: 1 was a moderate event of peripheral neuropathy and 1 was a severe event of bowel obstruction resulting from Meckel diverticulum.
Summary of AEs
| . | Patients (N = 157) . |
|---|---|
| Any patient with an AE, n (%)* | 147 (94) |
| Severity by event, number of events (%)* | |
| Mild | 1592 (74) |
| Moderate | 502 (23) |
| Severe | 62 (3) |
| Patients reporting at least 1 related AE, n (%) | 84 (54) |
| Patients reporting at least 1 serious AE, n (%) | 27 (17) |
| Patients with an AE leading to withdrawal, n (%) | 12 (8) |
| Deaths | 0 |
| Related AEs by patient in ≥5% of patients, n (%)† | 81 (53) |
| Abdominal pain upper | 11 (7) |
| Arthralgia | 9 (6) |
| Dyspepsia | 9 (6) |
| Fatigue | 8 (5) |
| Gastritis | 8 (5) |
| Diarrhea | 8 (5) |
| Nerve conduction studies abnormal | 8 (5) |
| . | Patients (N = 157) . |
|---|---|
| Any patient with an AE, n (%)* | 147 (94) |
| Severity by event, number of events (%)* | |
| Mild | 1592 (74) |
| Moderate | 502 (23) |
| Severe | 62 (3) |
| Patients reporting at least 1 related AE, n (%) | 84 (54) |
| Patients reporting at least 1 serious AE, n (%) | 27 (17) |
| Patients with an AE leading to withdrawal, n (%) | 12 (8) |
| Deaths | 0 |
| Related AEs by patient in ≥5% of patients, n (%)† | 81 (53) |
| Abdominal pain upper | 11 (7) |
| Arthralgia | 9 (6) |
| Dyspepsia | 9 (6) |
| Fatigue | 8 (5) |
| Gastritis | 8 (5) |
| Diarrhea | 8 (5) |
| Nerve conduction studies abnormal | 8 (5) |
Regardless of relationship to eliglustat.
Considered by investigator to be possibly, probably, or definitely related to eliglustat. Two related AEs in 2 patients were considered serious and possibly related to eliglustat (neither led to trial withdrawal): 1 peripheral neuropathy (moderate), 1 bowel obstruction from Meckel diverticulum (severe).
Discussion
The primary analysis from the ENCORE trial clearly showed that eliglustat was noninferior to imiglucerase in its capacity to maintain parameters indicating disease that was stable for 1 year.14 The analysis reported here, representing 511 patient-years of eliglustat exposure, shows long-term safety and tolerability along with stable disease parameters and maintenance of therapeutic goals for up to 4 years in the cohort of patients assigned to receive eliglustat and the original imiglucerase cohort who later moved to eliglustat treatment after participation in the first year of the trial. Mean absolute values for hemoglobin concentration, platelet count, spleen volume, liver volume, and lumbar spine and femur z scores remained stable over 4 years. Although, for the reasons previously set out, the number of patients available for analysis after 4 years of treatment under the constraints of a clinical trial was considerably reduced, the statistical analysis using a repeated-measures mixed model to detect linear trends identified modest but statistically significant improvements in spleen volume, liver volume, and lumbar spine z scores. These results should be interpreted cautiously because of the fewer patients from whom 4-year data are available, but analysis of the response parameters in this subset reveals the same long-term trends as in the entire trial cohort.
Biomarkers reflecting Gaucher disease activity were also found to be stable or showed modest improvement over time. Quality-of-life measures, which indicated a relatively mild burden of disease at baseline, also remained stable for up to 4 years in patients receiving eliglustat, indicating that the quality of life that they had achieved after a mean of 10 years on enzyme therapy was maintained while they were taking eliglustat therapy in the long term.
Only 4 patients (2.5%) withdrew from this long-term trial because of AEs considered to be treatment-related; no patient withdrew from the study because of any significant clinical deterioration. Most patients who did not meet the primary composite trial end point of stability relative to their baseline values remained clinically stable, as evidenced by the higher proportion of patients year to year who maintained absolute values within the prespecified thresholds for therapeutic goals (Figure 3). Because trial stability goals were defined relative to each patient’s individual baseline, patients could (and often did) fail to meet the composite end point because of a clinical value that did not signify deterioration. For example, a patient whose baseline liver volume increased from 0.9 MN at baseline to 1.1 MN would fail to meet the composite end point because this represents a >20% increase in liver volume, despite the fact that the actual value of 1.1 is well within established therapeutic goals for Gaucher patients. There were no apparent predictors of inability to meet the composite trial end point, including age, sex, and splenectomy status. Obesity was also evaluated because of the possibility that drug distribution could be lower in obese individuals.
No new safety concerns were identified in this long-term trial; this is consistent with a combined analysis of AE data from all 4 phase 2 and 3 eliglustat clinical trials.7 Only 7 types of AEs considered related to treatment by the investigator were reported in 5% or more of patients, with the most common being upper abdominal pain, reported in 7% of patients. Three of the 7 most common related AEs in the trial are also known to be common in Gaucher disease: fatigue, arthralgia, and abnormal nerve conduction studies. Of note, as in all clinical trials of eliglustat hitherto, we did not see the pattern of chronic diarrhea, weight loss, or new tremor, which led to high rates of drug discontinuation in clinical trials of miglustat (Zavesca; Actelion, Allschwil, Switzerland), the first commercially available substrate reduction therapy, approved as a second-line treatment of adults with Gaucher disease who are not candidates for enzyme therapy.18-20
Despite the salutary findings of this study, we recognize that there are a few inherent limitations. During the long-term extension phase of ENCORE, there was no comparator group because all patients were taking eliglustat, and thus no direct comparison can be made over this period with patients who continue to receive long-term enzyme therapy. Although it has been suggested that relapse is uncommon once patients with Gaucher disease type 1 have had their bulk disease controlled with enzyme therapy,21 the effects of the constrained supply of imiglucerase from 2009 through 2012 show that clinical deterioration does occur in as little as 3 months with treatment interruption.22,23 In our study, clinical stability was maintained with respect to hemoglobin concentration, platelet count, liver and spleen volumes, bone mineral density, and Gaucher biomarkers for up to 4 years, well beyond the interval that might be attributed to residual effects of prior long-term enzyme therapy. Although we are encouraged by the long-term maintenance of bone health, because the population had no active bone disease at baseline, data from this trial do not address the question of whether eliglustat can reverse preexisting bone disease.
Patients with Gaucher disease are known to be at increased risk of both Parkinson disease and hematologic malignancies.24,25 It will be important to evaluate the impact (if any) of eliglustat on incidence of these concomitant diseases in long-term observational studies; however, because the drug does not cross the blood–brain barrier, we would not expect a salutary effect of eliglustat on Parkinson disease.
Long-term eliglustat compliance was good, with 96% of patients taking at least 80% of their pills. Notably, this long-term analysis included all eliglustat-treated patients, unlike in the primary efficacy analysis, which, as a noninferiority trial, had excluded 2 patients whose study drug compliance was less than 80%. It will be important to monitor “real-world” compliance and its impact on drug efficacy because compliance rates are likely to decrease outside of a clinical trial setting.
As with many other oral drugs, eliglustat is metabolized primarily through the cytochrome P450 2D6 (CYP2D6) pathway. Dosing in the eliglustat trials was titrated, with the aim of maintaining a plasma trough level ≥5 ng/mL, at which half-maximal inhibition of glucosylceramide synthase is predicted to occur. However, subsequent pharmacokinetic analyses of clinical trial data established that the major determinant of plasma trough level of eliglustat was the patient’s CYP2D6-metabolizer status, and that achievement of the target 5 ng/mL level was not necessary for drug efficacy.26 Therefore, dosing in the drug label is based on the predicted CYP2D6 phenotype, as determined by genetic testing. The label also makes recommendations with regard to concomitant medications that might either inhibit efficacy of eliglustat or increase plasma drug levels.27,28 As in the general population,6 approximately 90% of patients with Gaucher disease are intermediate or extensive CYP2D6 metabolizers,7 with the remainder being poor metabolizers, ultrarapid metabolizers, or unknown. Of note, the ENCORE trial included 7 patients with an ultrarapid CYP2D6 metabolizer genotype, for whom eliglustat would be contraindicated in drug labels because of insufficient information about this small patient subgroup; all 7 of these patients met the primary composite end point throughout the trial.
We conclude that clinical stability, judged by composite and individual measures, was maintained in eliglustat-treated patients with Gaucher disease type 1 who remained in the ENCORE trial for up to 4 years. Eliglustat was generally well tolerated and no new or long-term safety concerns were identified.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and health care professionals who participated in this trial; Laurie LaRusso, ELS (Chestnut Medical Communications, Walpole, MA), for medical writing support funded by Sanofi Genzyme; and Yaoshi Wu (Sanofi Genzyme Biostatistics) and Lisa Underhill (Sanofi Genzyme Global Scientific Communications) for critical review of the manuscript.
The Study of Eliglustat Tartrate (Genz-112638) in Patients With Gaucher Disease Who Have Reached Therapeutic Goals With Enzyme Replacement Therapy was funded and conducted by Sanofi Genzyme. This work was supported by the UK Medical Research Council (MR/K015338/1) and National Institutes of Health Research, Cambridge Biomedical Research Centre (Metabolic theme) (T.M.C.).
Authorship
Contribution: T.M.C., G.D., R.C., M.B., T.A.B., A.M.M., E.L., B.R., O.G.-A., N.W., A.E.-B., P.S.K., and M.L.P. recruited patients and did the study research; T.M.C. contributed substantially to the manuscript; S.J.M.G. analyzed safety data; R.T. performed the biostatistical analyses; M.J.P. designed the study and analyzed study data; and all authors reviewed and approved initial and final versions of the manuscript.
Conflict-of-interest disclosure: T.M.C., G.D., R.C., M.B., T.A.B., A.M.M., E.L., B.R., O.G.-A., N.W., A.E.-B., and M.L.P. are principal investigators and have received honoraria from Sanofi Genzyme; P.S.K. was a principal investigator and subsequently a coprincipal investigator in the Study of Eliglustat Tartrate (Genz-112638) in Patients With Gaucher Disease Who Have Reached Therapeutic Goals With Enzyme Replacement Therapy (ENCORE) trial and has received honoraria from Sanofi Genzyme. T.M.C., M.B., E.L., B.R., O.G.-A., and P.S.K. have received travel reimbursement from Sanofi Genzyme. T.M.C. has been on the advisory board of Sanofi Genzyme. M.B., T.A.B., B.R., and P.S.K. are members of the North American advisory board for the International Collaborative Gaucher Group Gaucher Registry. T.A.B. has received honoraria from BioMarin. T.M.C., E.L., and P.S.K. have received honoraria and travel reimbursement from Shire. M.J.P. and S.J.M.G. are employees of Sanofi Genzyme and R.T. is a paid biostatistical consultant for Sanofi Genzyme.
The current affiliation for T.A.B. is Department of Pediatrics, Section of Genetics and Metabolism, Arkansas Children's Hospital, Little Rock, AR.
Correspondence: Timothy M. Cox, University of Cambridge, Department of Medicine, Box 157, Level 5, Addenbrooke's Hospital, Cambridge CB2 0QQ, United Kingdom; e-mail: tmc12@medschl.cam.ac.uk.