In this issue of Blood, Yoshizato et al performed genomic analysis on 797 patients with myelodysplastic syndrome (MDS) or secondary acute myeloid leukemia (sAML) who received unrelated bone marrow transplants (BMTs) and showed that TP53 mutations combined with complex karyotypes (CKs) or RAS-pathway mutations in the setting of preexisting myeloproliferative neoplasms (MPNs) conferred poor outcomes.1
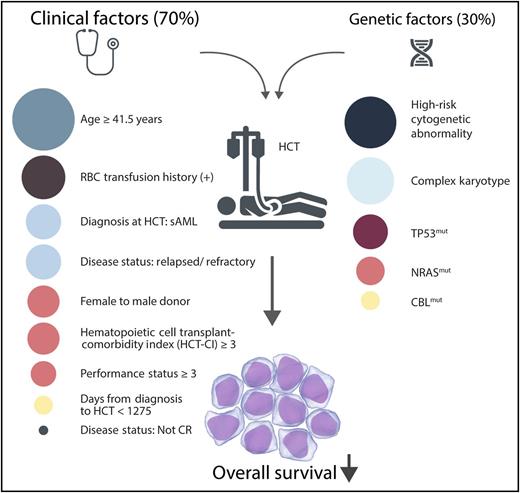
The relative weighted contributions (shown by circle size) of significant clinical factors and genomic factors that predict worse survival chiefly because of leukemic relapse after BMT for MDS and sAML. CR, complete response; HCT, hematopoietic cell transplant; mut, mutation; RBC, red blood cell count. Professional illustration by Somersault 18:24.
The relative weighted contributions (shown by circle size) of significant clinical factors and genomic factors that predict worse survival chiefly because of leukemic relapse after BMT for MDS and sAML. CR, complete response; HCT, hematopoietic cell transplant; mut, mutation; RBC, red blood cell count. Professional illustration by Somersault 18:24.
Myeloid neoplasms including MDS and AML represent an ongoing therapeutic challenge. For lower-risk MDS patients with low blast percentages, well-preserved hematopoiesis, and favorable cytogenetics, careful clinical monitoring is recommended. Similarly, for AML patients with more favorable cytogenetic abnormalities (eg, t(15;17), inv16, t(8;21)), induction therapy followed by a series of consolidation treatments is usually sufficient to induce long-term remissions. For most other MDS and AML patients, and especially those with sAML or treatment-related AML, allogeneic stem cell transplant (SCT) offers the best chance for cure. With the advent of reduced-intensity conditioning2 and posttransplant high-dose cyclophosphamide for haploidentical related donors,3 the improved safety and expanded donor pool has made allogeneic SCT a potential option for most patients with MDS or AML. Allogeneic SCT is also a platform for targeted agents or immunotherapeutics4,5 that enhance the allogeneic graft-versus-leukemia effect and eliminate residual or relapsed malignancy. It is therefore beneficial to identify which patients are at greater risk for treatment failure from relapse after allogeneic SCT.
Although karyotyping remains the most important diagnostic and prognostic tool, advanced genomic analytics such as high-density comparative genomic hybridization, single nucleotide polymorphism (SNP) arrays covering the entire genome, targeted sequencing of specific genes, and massively parallel sequencing efforts have led to the discovery of other pathogenic and prognostic mutations involving genes such as FLT3, NRAS, IDH1, and DNMT3A.6 Among the lessons from these landmark studies were that AML genomes contain hundreds of somatic mutations, the vast majority of which are age dependent, benign passenger events that are captured by a small number of pathogenic and clonogenic driver mutations. This paradigm offers hope that identification of the few key pathogenic driver and cooperative mutations in each case of AML could lead to a personalized therapeutic approach.
Yoshizato et al studied 797 patients with MDS (including 6% with MDS/MPNs and 24% with subsequent transformation to sAML) who received unrelated allogeneic BMTs through the Japan Marrow Donor Program. By using targeted-capture sequencing with a complementary RNA bait library for known driver mutations in 69 genes and for 1,158 SNPs that spanned the genome, the authors sought to identify which specific driver mutations and copy-number abnormalities could help predict BMT outcomes. In univariate analysis, 4 mutations and 9 copy-number alterations present in >5% of the patients were significantly associated with post-SCT survival. On multivariate analysis in combination with clinical factors, 4 genetic lesions, including mutations in NRAS, TP53, and CBL, along with CK (by conventional cytogenetics as well as ≥3 abnormal copy-number segments by SNP array) were associated with worse outcomes (see figure). Significant clinical factors that were identified by the model included performance status (≥3), age at transplant (≥41.5 years), hematopoietic cell transplant–specific comorbidity index (score ≥3), female donor to male recipient, diagnosis of sAML, and disease status at transplant. Interestingly, the set of prognostically important gene mutations identified in the study by Yoshizato et al differed from those identified in previous studies that included mutations in DNMT3A, TET2, RUNX1, and ASXL1.7,8 These differences may be attributed to the retrospective nature of these studies, differences in patient selection criteria and disease subtypes, and specific inclusion of an MDS/MPN subtype in the Yoshizato et al study.
Further modeling revealed an important interaction between TP53 mutations and CKs such that the combination of TP53 mutation and CK was associated with a median survival of only 4.8 months posttransplant mainly because of early relapse. The outcome for TP53-mutated MDS/sAML without CK was significantly better, with 73% of such patients alive at 5 years. Similarly, patients carrying mutations in RAS-pathway genes (including NRAS, CBL and, less frequently, KRAS, NF1, and PTPN11, altogether found in 17% of the 797 patients) were associated with worse survival but chiefly in the subgroup of patients with preexisting MDS/MPN. The clinical impact of specific gene mutations can be modified by the presence or absence of other genetic lesions, as demonstrated for FLT3-ITD wherein the adverse impact of this mutation on survival is dramatically enhanced by the simultaneous presence of both DNMT3A and NPM1 mutations.9 Similarly, patients with coexisting MLL-PTD and FLT3-TKD mutations are associated with a worse outcome than when either mutation is present alone.
Molecular genetic analysis has not yet been routinely incorporated into pre-SCT risk stratification schemes for posttransplant management of patients with MDS/AML. More data are needed to establish which somatic driver and cooperating mutations serve as good predictive markers of response to SCT, and the article by Yoshizato et al is an important contribution to this effort. Conceivably, a poor-risk mutational profile can help guide the need for post-SCT maintenance therapies to prevent relapse, such as hypomethylating agents, immunomodulatory agents including lenalidomide and checkpoint inhibitors, or mutation-specific targeted therapies including FLT3 or RAS-pathway inhibitors. For example, in a study of 37 patients, maintenance azacitidine initiated at a median of 54 days post-SCT led to 1- and 2-year overall survival (OS) of 81% and 49%, respectively, with 1- and 2-year relapse-free survival (RFS) of 57% and 49%, respectively, with no extensive chronic graft-versus-host disease (cGVHD).10 Post-SCT maintenance therapy using sorafenib may also benefit patients with FLT3-ITD mutations, as shown in a study of 22 FLT3-ITD–mutant AML patients, yielding a 1-year RFS of 85%, OS of 95%, and 38% incidence of cGVHD at a median follow-up of 16.7 months post-SCT.5
When combined with clinical factors, the most significant genetic abnormalities of mutations in TP53, CBL, and NRAS, along with CK and high-risk cytogenetics by conventional testing, still accounted for only 30% of the variation in transplant survival outcome. Although genomic analysis has great potential to optimize and personalize the delivery of effective therapy, for now, patient and disease-related clinical factors remain the major prognostic component of the posttransplant treatment algorithm.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal