In this issue of Blood, Bernitz et al show that granulocyte colony-stimulating factor (G-CSF) selectively mobilizes nondividing hematopoietic stem cells (HSCs).1 A majority of the HSCs remain quiescent after mobilization and ultimately return to the bone marrow without dividing. Thus, G-CSF does not uniformly drive adult HSCs into cycle.
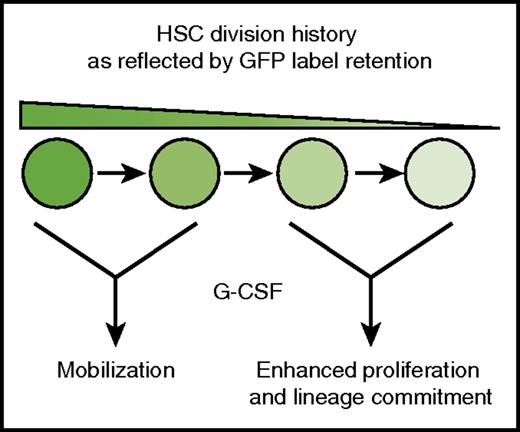
HSCs show a nonuniform response to G-CSF. G-CSF selectively mobilizes infrequently dividing HSCs (high GFP expression), and it induces proliferation and myeloid commitment in HSCs with more extensive division histories (low GFP expression).
HSCs show a nonuniform response to G-CSF. G-CSF selectively mobilizes infrequently dividing HSCs (high GFP expression), and it induces proliferation and myeloid commitment in HSCs with more extensive division histories (low GFP expression).
G-CSF is widely used in the clinical setting to mobilize adult HSCs for autologous or allogeneic stem cell transplants. Before mobilization, most HSCs are quiescent. In mice, lineage tracing studies have shown that a subset of adult HSCs divide very infrequently and therefore undergo very few self-renewing division cycles throughout life.2-4 This pool of “dormant” HSCs has been shown to repopulate transplant recipients more effectively than HSCs with extensive division histories.2-4 This suggests that HSC function declines with increasing cumulative divisions. G-CSF has been shown to induce HSC proliferation before mobilization, and HSC function is compromised for at least a short period of time after G-CSF treatment.5,6 These observations raise the question of whether the decline in HSC repopulating activity observed after G-CSF treatment is a direct consequence of enhanced proliferation, particularly within the otherwise dormant HSC pool.
Bernitz et al used lineage tracing to test whether G-CSF treatment depletes the dormant HSC pool. They used a human CD34-tTA transgene to drive expression of green fluorescent protein–tagged histone H2B (H2B-GFP) in adult HSCs.4 They then administered doxycycline to adult mice to suppress H2B-GFP expression. In that model, GFP signal intensity declined with each cell division cycle, and the infrequently dividing dormant HSCs could be identified as a GFP label–retaining subpopulation (hereafter called LR-HSCs). As expected, 4 days of G-CSF treatment caused a severe reduction in bone marrow LR-HSCs, but surprisingly, this effect was transient. When mice were analyzed 8 weeks after G-CSF treatment, LR-HSC numbers were equivalent in treated and control mice. Subsequent analysis showed that the majority of HSCs found in the peripheral blood after 4 days of treatment with G-CSF were LR-HSCs, in contrast to bone marrow HSCs. These results show that G-CSF has different short-term effects on the label-retaining and non–label-retaining subpopulations of phenotypic HSCs. LR-HSCs mobilize to the periphery without dividing whereas non–LR-HSCs remain in the bone marrow and proliferate (see figure).
Because bone marrow LR-HSCs repopulate more efficiently than non–LR-HSCs, one might expect that mobilized HSCs would exhibit robust repopulating activity. Bernitz et al tested this possibility with an elegant mixing study in which mobilized HSCs were competed with nonmobilized bone marrow HSCs with comparable division histories. They found that bone marrow HSCs were approximately 4 times more effective at repopulating primary and secondary transplant recipients, even after normalizing for label retention. This affirms previous studies showing that mobilization transiently compromises HSC function,5 but it also shows that the compromise is not due to a loss of quiescence.
The authors next explored the fate of the non–LR-HSCs that proliferate and remain in the bone marrow after G-CSF treatment. They analyzed subpopulations of phenotypic HSCs based on CD41 expression, a marker of myeloid-biased HSCs.7 They found that G-CSF induced proliferation within the CD41+, but not the CD41–, subpopulation. Furthermore, the non–LR-HSC fraction was enriched for CD41+ and CD41Hi cells. Competitive transplantation assays showed that after G-CSF treatment, CD41+ and CD41Hi HSCs had minimal repopulating activity. Most CD41– HSCs had only short-term repopulating activity after G-CSF treatment. Altogether, these data suggest a model in which HSCs with long-term repopulating activity are mobilized after G-CSF treatment. Phenotypic HSCs that remain in the bone marrow either have limited self-renewal capacity due to an extensive cumulative division history or are committed progenitors.
The model presented by Bernitz et al ties together many earlier observations. It explains why bone marrow HSCs seem to be severely compromised immediately after G-CSF treatment.6,8 Furthermore, the article helps resolve a paradox that surrounds the effects of G-CSF on HSC self-renewal. Many hematopoietic stresses, such as blood loss, infection, inflammation, or chemotherapy, can induce HSC proliferation.3,9 In those cases, sustained proliferation correlates with a decline in functional HSCs that is often described as “HSC exhaustion.” Although G-CSF has been shown to induce HSC proliferation and transiently impair HSC function, it does not appear to permanently deplete the HSC pool: cessation of the cytokine allows bone marrow HSC function to return to normal.8 An explanation for this difference, provided herein, is that G-CSF–stimulated LR-HSCs do not actually divide extensively, and proliferation is largely restricted to non–self-renewing populations. This finding aligns well with a recent study by Kovtonyuk et al,10 and it is good news from a clinical perspective because it suggests that G-CSF treatment will not deplete the dormant HSC pool in human stem cell transplant donors.
The Bernitz et al study does raise additional questions. It is not clear why LR-HSCs mobilize more efficiently than non–LR-HSCs. The authors suggest proximity to the vasculature as one potential mechanism. Differences in the expression of surface proteins that regulate mobilization and homing (eg, VLA-4 or CXCR4) might also contribute. It is also not clear why LR-HSCs are refractory to the mitogenic effects of G-CSF or why some committed progenitors (eg, most colony-forming progenitors) mobilize efficiently, whereas other committed progenitors (eg, CD41+ phenotypic HSCs) do not. Finally, the study raises the question of whether other mobilizing regimens, such as AMD3100, will also selectively mobilize LR-HSCs. Although further studies are needed to address these questions, the study by Bernitz et al provides an important framework for understanding how G-CSF regulates mobilization and proliferation in subpopulations of phenotypic HSCs. The concepts presented in their article will shape future efforts to improve HSC mobilization regimens in humans.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal