To the editor:
An understanding of hematopoietic stem cell (HSC) biology is therapeutically important because of the many human diseases that result from errors in HSC regulation or that can be treated with HSC transplantation. The zebrafish is a powerful model for studying HSC biology in vivo1-3 ; however, monoclonal antibodies are challenging to generate for use with zebrafish hematopoietic cells.4 Here, we report the development and characterization of a monoclonal antibody to zebrafish Cd41 and show that it can be used to negatively enrich for HSCs.
Zebrafish Cd41, also known as GpIIb and Itga2b, is a cell-surface protein expressed at high levels on thrombocytes and at lower levels on HSCs.5-7 In humans and other species, CD41 forms cell-surface heterodimers with CD61, also known as GPIIIa or ITGB3. The CD41/CD61 (GPIIb/IIIa) heterodimer plays a critical role in platelet activation, and deficiencies in its function cause the human bleeding disorder Glanzmann thrombasthenia.8,9 Previous work has shown that Tg(cd41:EGFP) zebrafish can be used to positively enrich for HSCs.6,10 In adult zebrafish, cd41:EGFPLow cells in kidney marrow have HSC activity, whereas cd41:EGFPHigh cells in peripheral blood are thrombocytes.6 These data suggested that an antibody to Cd41 could be useful for studying the zebrafish thrombocyte lineage and for isolating zebrafish HSCs.
To generate a monoclonal antibody to zebrafish Cd41, in 293T cells we expressed a secreted form of zebrafish Cd41/mouse CD61 in which the transmembrane domains of each protein were replaced by an acid peptide and base peptide, respectively, to promote leucine zipper-mediated heterodimerization and secretion. The heterodimeric protein was then used as an immunogen in the production of mouse hybridomas (see supplemental Figure 1A, available on the Blood Web site). Hybridomas were screened using a cell line expressing native zebrafish Cd41 and zebrafish Cd61b. The Cd61b was FLAG-tagged to allow confirmation of heterodimer expression on the cell surface without altering the Cd41 epitope. This yielded a reactive hybridoma that was selected for further subcloning and characterization (supplemental Figure 1B).
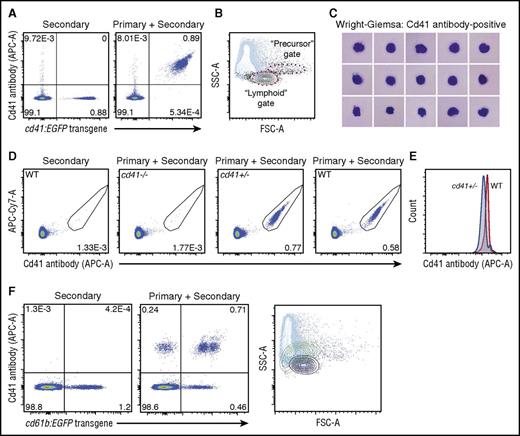
We initially tested the Cd41 antibody by flow cytometry using peripheral blood from adult Tg(cd41:EGFP) zebrafish. This revealed that the Cd41 antibody recognizes cd41:EGFP-positive cells with greater than 99% sensitivity and specificity (Figure 1A). Based on light scatter characteristics, these cells are predominantly located in the expected region,5 which has traditionally been termed the “lymphoid” gate (Figure 1B).11 At least 2 discrete populations of double-positive cells are discernable; the most abundant of these is relatively restricted to the lymphoid gate. Cytopreparations of cells sorted by flow cytometry demonstrated that Cd41 antibody-positive cells are predominantly thrombocytes, as anticipated (Figure 1C). Furthermore, RNA sequencing confirmed that the most highly expressed genes in antibody-positive cells/zebrafish thrombocytes are well known to be expressed by human and mouse platelets and that there is significant evolutionary conservation among the 3 species (supplemental Tables 1 and 2; supplemental Figure 2). As an alternative method to flow cytometry, the characteristics of the Cd41 antibody were assessed by immunofluorescence (supplemental Figure 3); congruent with the flow cytometry results, the Cd41 antibody stained cells in immunofluorescence experiments with greater than 95% sensitivity and specificity.
The Cd41 antibody binds cd41:EGFP-positive cells in zebrafish peripheral blood with >99% sensitivity and specificity and is specific for Cd41. (A) The Cd41 antibody recognizes cd41:EGFP-positive cells in zebrafish peripheral blood with >99% sensitivity and specificity by flow cytometry. (B) Light scatter characteristics demonstrate that double-positive cells (black) are mostly located within the “lymphoid” gate (red dashed line) when overlaid on live cells (blue). Contour levels are set at 10% and outliers are shown. The “precursor” gate is also shown for reference (purple dashed line). (C) Wright-Giemsa stain of cytopreparation showing that Cd41 antibody-positive cells are generally thrombocytes based on morphology. (D) Flow cytometry of zebrafish peripheral blood reveals a loss of Cd41 antibody staining in cd41 mutant zebrafish. There is no reduction in the percentage of antibody-stained Cd41-positive cells in heterozygous zebrafish. (E) The median fluorescence intensity of cells from heterozygous zebrafish is about two-thirds that of wild-type (WT) zebrafish. (F) In spleen cells from adult zebrafish, cd61b:EGFP is expressed by a subset of Cd41 antibody-positive cells, but populations of both cd61b:EGFP single-positive and Cd41 antibody single-positive cells also exist. By light scatter characteristics, the cd61b:EGFP single-positive cells (green) map to a slightly different location than the overlapping double-positive cells (black) and Cd41 antibody single-positive cells (purple). Live cells are shown in the background (blue). Contour levels are set at 10% and outliers are shown. APC-A, allophycocyanin-area; FSC-A, forward scatter-area; SSC-A, side scatter-area.
The Cd41 antibody binds cd41:EGFP-positive cells in zebrafish peripheral blood with >99% sensitivity and specificity and is specific for Cd41. (A) The Cd41 antibody recognizes cd41:EGFP-positive cells in zebrafish peripheral blood with >99% sensitivity and specificity by flow cytometry. (B) Light scatter characteristics demonstrate that double-positive cells (black) are mostly located within the “lymphoid” gate (red dashed line) when overlaid on live cells (blue). Contour levels are set at 10% and outliers are shown. The “precursor” gate is also shown for reference (purple dashed line). (C) Wright-Giemsa stain of cytopreparation showing that Cd41 antibody-positive cells are generally thrombocytes based on morphology. (D) Flow cytometry of zebrafish peripheral blood reveals a loss of Cd41 antibody staining in cd41 mutant zebrafish. There is no reduction in the percentage of antibody-stained Cd41-positive cells in heterozygous zebrafish. (E) The median fluorescence intensity of cells from heterozygous zebrafish is about two-thirds that of wild-type (WT) zebrafish. (F) In spleen cells from adult zebrafish, cd61b:EGFP is expressed by a subset of Cd41 antibody-positive cells, but populations of both cd61b:EGFP single-positive and Cd41 antibody single-positive cells also exist. By light scatter characteristics, the cd61b:EGFP single-positive cells (green) map to a slightly different location than the overlapping double-positive cells (black) and Cd41 antibody single-positive cells (purple). Live cells are shown in the background (blue). Contour levels are set at 10% and outliers are shown. APC-A, allophycocyanin-area; FSC-A, forward scatter-area; SSC-A, side scatter-area.
To confirm that the Cd41 antibody recognizes zebrafish Cd41, we tested the antibody against a cd41 mutant zebrafish (itga2bsa10134) that is expected to lack expression of Cd41.12 Homozygous mutant zebrafish are observed at much less than the expected frequency in heterozygous crosses but have circulating thrombocytes. Analysis of peripheral blood from cd41−/− zebrafish by flow cytometry revealed a loss of Cd41 antibody staining (Figure 1D). The proportion of peripheral blood cells stained was normal in heterozygous mutant zebrafish (Figure 1D), but the median fluorescent intensity of heterozygous cells was two-thirds that of the wild-type cells (Figure 1E). This is consistent with decreased cell-surface expression of Cd41 in heterozygous cells. Because loss of Cd41 at the cell surface might compromise Cd61b cell-surface expression, we confirmed that the Cd41 antibody binds to Cd41 and not Cd61b in the Cd41/Cd61b heterodimer using adult Tg(cd61b:EGFP) zebrafish (Figure 1F).
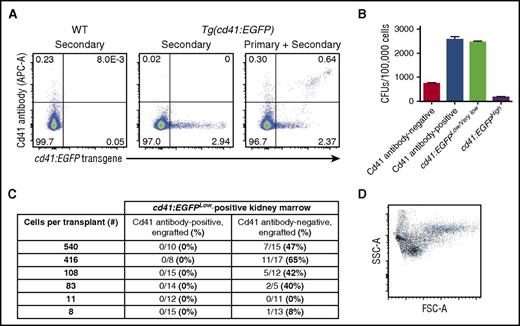
Having characterized the Cd41 antibody for use with adult zebrafish peripheral blood, we turned our attention to adult zebrafish kidney marrow. In kidney marrow, the Cd41 antibody identifies a subset of cd41:EGFP-positive cells, specifically the population with higher cd41:EGFP expression (Figure 2A). Multiple possibilities for this differential binding exist, including transgene activation in the absence of transcription at the endogenous locus and production of discrete epitopes in the 2 cell groups. Because some Cd41 antibody-positive cells isolated from kidney marrow in the absence of a significant forward scatter size limit appeared to be immature when examined using cytopreparations, and because a minority are located within the so-called “precursor” gate, we performed colony-forming assays to determine if some of these cells were progenitor cells that had not yet terminally differentiated. This revealed that Cd41 antibody-positive cells exhibit colony-forming activity comparable to what is obtained with cd41:EGFPLow/Very Low-positive cells (Figure 2B). In this case, the lower level of colony-forming activity in the Cd41 antibody-negative cell population reflects the gating strategy, which includes all Cd41 antibody-negative cells: although HSCs and progenitors such as common myeloid progenitors, common lymphoid progenitors, and granulocyte-monocyte progenitors would be included, abundant terminally differentiated cells such as mature myeloid cells, lymphoid cells, and red blood cells are also included. As expected, cd41:EGFPHigh cells do not form significant numbers of colonies because they are largely mature thrombocytes.5,6
The Cd41 antibody binds thrombocytes and thrombocyte precursors in zebrafish kidney marrow and permits HSC enrichment. (A) The Cd41 antibody binds cd41:EGFPHigh-positive cells (including thrombocytes) preferentially in kidney marrow. (B) Colony-forming assays suggest that Cd41 antibody-positive cells in kidney marrow include thrombocyte precursors. (C) Transplantation of sorted cd41:EGFPLow kidney marrow cells reveals that the Cd41 antibody-positive cell fraction does not engraft, but that the Cd41 antibody-negative cell fraction does. (D) Representative flow cytometry plot from the kidney marrow of a transplant recipient at 3 months posttransplant. Multilineage engraftment is apparent based on the location of ubi:mCherry-positive cells (black) relative to live cells (blue) by light scatter characteristics.
The Cd41 antibody binds thrombocytes and thrombocyte precursors in zebrafish kidney marrow and permits HSC enrichment. (A) The Cd41 antibody binds cd41:EGFPHigh-positive cells (including thrombocytes) preferentially in kidney marrow. (B) Colony-forming assays suggest that Cd41 antibody-positive cells in kidney marrow include thrombocyte precursors. (C) Transplantation of sorted cd41:EGFPLow kidney marrow cells reveals that the Cd41 antibody-positive cell fraction does not engraft, but that the Cd41 antibody-negative cell fraction does. (D) Representative flow cytometry plot from the kidney marrow of a transplant recipient at 3 months posttransplant. Multilineage engraftment is apparent based on the location of ubi:mCherry-positive cells (black) relative to live cells (blue) by light scatter characteristics.
Because the cd41:EGFPHigh population is enriched for thrombocytes and the cd41:EGFPLow population is enriched for HSCs,6 we sought to determine whether the Cd41 antibody permits enrichment of adult zebrafish HSCs by negative selection. We performed limiting dilution transplantation experiments using kidney marrow from Tg(cd41:EGFP;ubi:mCherry) zebrafish that was sorted into a cd41:EGFPLow, Cd41 antibody-positive fraction and a cd41:EGFPLow, Cd41 antibody-negative fraction. The sorting strategy included a restriction on forward scatter and used splenocytes to help more rigorously define the cd41:EGFPHigh population (supplemental Figure 4A). Morphologically, the Cd41 antibody-positive fraction contained thrombocytes (supplemental Figure 4B) and the Cd41 antibody-negative fraction contained cells that were mostly immature with high nuclear to cytoplasmic ratios (supplemental Figure 4C). These fractions were transplanted into irradiated recipients and engraftment was assessed at 3 months posttransplant. Engraftment was never obtained from cd41:EGFPLow, Cd41 antibody-positive fractions; by contrast, engraftment was frequently obtained from cd41:EGFPLow, Cd41 antibody-negative fractions (Figure 2C). Engraftment was multilineage based on expression of the ubi:mCherry transgene in erythroid, myeloid, precursor, and lymphoid gates by flow cytometry (Figure 2D).
The Cd41 antibody characterized in this study is the first monoclonal antibody to permit HSC enrichment by flow cytometry in zebrafish. Limiting dilution software suggests that the frequency of HSCs in our cd41:EGFPLow, Cd41 antibody-negative fraction is 1/456 (302-688) but this is likely an underestimate resulting at least in part from transplantation being performed without matching major histocompatibility loci between donors and recipients. Our results support the broad use of the Cd41 antibody with other transgenic zebrafish lines in the quest to purify zebrafish HSCs. Indeed, the Cd41 antibody represents a first step toward developing a set of zebrafish “lineage-negative” markers that permit the enrichment of HSCs, as is already done in mice and humans.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The cd61b:EGFP transgenic line, formally named Tg(itgb3:EGFP)pd66, was a kind gift of Jinhu Wang and Kenneth Poss. The authors thank Ron Mathieu and the personnel of the Children’s Hospital and Harvard Stem Cell Institute Flow Cytometry Research Facility core for advice on flow cytometry and cell sorting, Ed Greenfield and the personnel of the Dana-Farber Cancer Institute Monoclonal Antibody Core for help generating the Cd41 antibody, Michelle Lin for assistance with western blotting and protein purification, Emily Hsieh for help with the immunofluorescence experiments, and Yi Zhou and Song Yang for bioinformatics and other support.
This work was supported in part by funding from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI; grants R01HL048801, P01HL32262-32, and U01HL100001-05) and NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grants P30DK49216 and R24DK092760) (L.I.Z.); T32 training grants from the NIH, National Cancer Institute (grant T32CA009172-39) and the NIH, NHLBI (grant T32HL116324-03) (J.M.G.); and the NIH, NIDDK (grant K01DK087814-01A1) (D.L.S.).
Contribution: J.M.G. performed fluorescence-activated cell sorting (FACS) experiments, transplantation, cytopreparations, immunofluorescence, and RNA sequencing; generated the first draft of the manuscript; and incorporated feedback from the other authors into subsequent drafts of the manuscript. A.D.L. was responsible for generating the Cd41 antibody and performed FACS experiments. M.S. performed the bioinformatics analyses. M.C.B. performed immunofluorescence experiments and helped with transplantation experiments. M.B.A. helped with transplantation experiments. E.M.D. helped with the generation of the Cd41 antibody. B.B. helped with the generation of the Cd41 antibody. R.I.H. provided technical advice. D.L.S. performed the colony-forming assay. C.L. provided technical advice and reagents for expressing secreted heterodimers of Cd41 and CD61. T.A.S. provided technical advice and reagents for expressing secreted heterodimers of Cd41 and CD61. L.I.Z. guided the research and edited the manuscript.
Conflict-of-interest disclosure: L.I.Z. is a founder and/or on the board of directors and has equity in Fate Therapeutics, Marauder Therapeutics, and Scholar Rock. The remaining authors declare no competing financial conflicts.
Correspondence: Leonard I. Zon, Boston Children's Hospital, Karp Research Building, 7th Floor, 300 Longwood Ave, Boston, MA 02115; e-mail: zon@enders.tch.harvard.edu.
References
Author notes
J.M.G. and A.D.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal