In this issue of Blood, Shafat et al describe an intriguing interplay between leukemic cells and adipocytes. Leukemic cells alter the metabolic state of adipocytes by increasing the levels of fatty acid binding protein-4 (FABP4) and enhancing lipolysis, which in turn fuels β-oxidation of fatty acids in leukemic cells thereby enhancing their survival and transformation potential.1
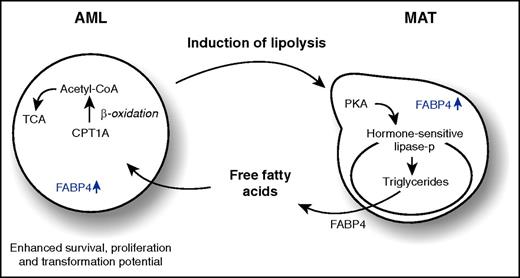
AML blasts enhance lipolysis of adipocytes in an FABP4-dependent manner, resulting in an enhanced secretion of free fatty acids that drive β-oxidation in AML cells and facilitate enhanced survival, proliferation, and transformation potential. Acetyl-CoA, acetyl coenzyme A; CPT1A, carnitine palmitoyltransferase 1A; MAT, marrow adipose tissue; lipase-p, phosphorylated lipase; TCA, tricarboxylic acid cycle.
AML blasts enhance lipolysis of adipocytes in an FABP4-dependent manner, resulting in an enhanced secretion of free fatty acids that drive β-oxidation in AML cells and facilitate enhanced survival, proliferation, and transformation potential. Acetyl-CoA, acetyl coenzyme A; CPT1A, carnitine palmitoyltransferase 1A; MAT, marrow adipose tissue; lipase-p, phosphorylated lipase; TCA, tricarboxylic acid cycle.
Hematopoietic stem cells (HSCs) reside within specialized niches in the bone marrow that can be composed of a large variety of different cell types.2 These include mesenchymal stromal cells and descendants thereof such as osteoblasts, chondrocytes, and adipocytes. HSC-derived cells such as macrophages, megakaryocytes, regulatory T cells, and even neural cells can reconstitute important components of the hematopoietic stem cell niche as well. The exact molecular mechanisms by which the niche has an impact on stem cell fate are far from resolved, but data so far have shown that quiescence, survival, and differentiation decisions of stem cells heavily depend on cues they receive from the bone marrow microenvironment.
Conversely, the niche can be remodeled by HSCs themselves, and in particular by leukemic cells. Typical consequences such as bone loss and altered angiogenesis have been reported in acute myeloid leukemia (AML) and myelodysplastic syndromes. By secreting proinflammatory cytokines, malignant cells can create a milieu in which survival and transformation of (pre)leukemic cells are facilitated at the expense of normal hematopoiesis.3,4
The current work by Rushworth and colleagues1 adds another twist to the story. They convincingly show that AML blasts store more lipids than normal CD34+ cells, that these lipids can actively be transferred from adipocytes to AML cells, and that survival is better maintained when AML cells are grown on adipocytes. In fact, coculture of AML blasts with adipocytes, or simply by treating adipocytes with AML-conditioned medium, increases their fatty acid release, coinciding with an activation of hormone-sensitive lipase, which activates lipolysis (see figure). This fatty acid release then drives β-oxidation in AML cells involving CPT1A. Oxygen consumption rates (OCRs) and survival of AML blasts increase when grown on adipocytes in a CPT1A-dependent manner. In addition, knockdown of CPT1A reduces the engraftment kinetics of primary AML samples in xenograft mice. Normal CD34+ cells are also able to induce lipolysis in adipocytes, but to a lesser extent when compared with AML blasts, and knockdown of CPT1A does not have an impact on the OCR of normal CD34+ cells.
To better understand the underlying mechanisms, Shafat et al show that upon coculturing AML blasts with adipocytes, FABP4 is upregulated in adipocytes at the messenger RNA (mRNA) level, coinciding with increased FABP4 protein secretion, presumably because FABP4 is exported out of the adipocyte when bound to lipids. This is functionally relevant because inhibition of FABP4 in adipocytes by a short hairpin RNA approach or by the small molecule inhibitor BMS309403 prevents AML proliferation on adipocytes but has no effect on normal CD34+ proliferation. Although the FABP4 secreted by adipocytes is not taken up by AML blasts directly, FABP4 mRNA levels are also upregulated in AML blasts themselves, in particular when cocultured on adipocytes. Although molecular mechanisms underlying this FABP4 upregulation remain to be clarified, knockdown of FABP4 in a Hoxa9/Meis1-driven murine leukemia model significantly prolongs survival.
The study by Shafat et al puts forth 2 important insights. First, AML blasts can have a direct impact on the niche by inducing lipolysis in adipocytes, and second, this release of free fatty acids then drives β-oxidation in AML cells, resulting in enhanced survival, proliferation, and transformation potential. These observations are in line with what has been observed in other solid tumors in which adipocytes have been shown to influence metastasis and survival and might also underlie in part the observation that obese leukemia patients have a poorer prognosis.5
Yet some questions remain. For instance, what are the critical components of the AML secretome that drive lipolysis of adipocytes? Recently, Ye et al6 showed by using a murine blast crisis chronic myeloid leukemia model that a subset of leukemia stem cells (LSCs) can reside in gonad adipose tissue where they are located adjacent to adipocytes. These LSCs are positive for the fatty acid transporter CD36 and express high levels of inflammatory cytokines, which in turn induce lipolysis in adipose tissue, and these LSCs are more resistant to chemotherapy. The induction of a local pro-inflammatory milieu might facilitate lipolysis in AML as well.
Why are normal CD34+ cells less efficient in inducing lipolysis? It is quite possible that this relates to the increased secretion of inflammatory cytokines and/or other factors specific to AML cells. But maybe even more intriguing, why would normal cells not make use of the release of free fatty acids to fuel β-oxidation as AML blasts do? In this context, it is also remarkable that ablation of marrow adipose tissue actually promotes hematopoietic recovery after irradiation, suggesting that adipocytes act as negative regulators for normal hematopoiesis.7 Future studies aimed at unraveling the metabolic hardwiring of leukemic versus normal hematopoietic cells will need to provide further insight into these issues.
Finally, although the work of Shafat et al provides a convincing case for the leukemic blast population, one wonders what happens at the more immature leukemic stem cell level. In a series of recent articles, it was shown that HSCs keep their mitochondrial activity at low rates to ensure quiescence, a glycolytic state, and self-renewal properties,8,9 and similar phenotypes have been described for LSCs.10 It is not clear how to reconcile fatty acid oxidation (FAO)–driven oxygen consumption resulting in enhanced adenosine triphosphate production and cell cycle progression with maintenance of stem cell fate. Potentially, FAO drives mitochondrial clearance instead of increasing OCR in the most primitive stem cell populations, as was recently shown for a population of Tie+ murine HSCs in which the FAO pathway promotes self-renewing expansion,8 and future studies will no doubt be aimed at further clarifying these phenomena.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal