Key Points
Mice deficient for basophil tryptase mMCP-11 showed ameliorated IgE-mediated allergic inflammation with reduced leukocyte infiltration.
This is the first demonstration that the basophil-derived protease plays a crucial role in allergic inflammation.
Abstract
Recent studies have identified nonredundant roles for basophils in immune responses including allergy and protective immunity. It is well known that activated basophils release granule contents such as histamine and proteases as do mast cells. However, the functional significance of basophil-derived proteases remains poorly understood in contrast to those released from mast cells. For this study we generated a line of knockout (KO) mice deficient for mouse mast cell protease-11 (mMCP-11) that is preferentially expressed by basophils rather than mast cells. In spite of normal development of basophils, the mMCP-11–deficient mice showed amelioration of immunoglobulin E–mediated chronic allergic inflammation (IgE-CAI), with reduction of cutaneous swelling, microvascular permeability, and leukocyte infiltration in the skin lesion, when KO mice were compared with wild-type mice. Repeated administration of recombinant mMCP-11 in the skin induced infiltration of leukocytes, including basophils, in a tryptase activity–dependent manner. The transwell migration assay in vitro suggested that mMCP-11–mediated proteolytic products of serum protein promoted migration of basophils, eosinophils, and macrophages via 1 or more G protein–coupled receptors. Thus, basophil tryptase mMCP-11 is a crucial effector molecule for the induction of IgE-CAI. This is the first demonstration that the basophil-derived protease plays a significant role in vivo.
Introduction
Basophils are the rarest type of granulocyte and represent less than 1% of peripheral blood leukocytes. They were often considered erroneously a minor relative or blood-circulating precursor of tissue-resident mast cells, which they resemble.1-3 It is now well accepted that basophils and mast cells are distinct cell lineages.4 Recent studies have identified crucial roles for basophils, distinct from those played by mast cells, in immune responses including allergy and protective immunity.5-8 In response to various stimuli, basophils can readily secrete large quantities of the cytokine interleukin-4 (IL-4), which plays a versatile role in immunity through regulating other cells.9 In contrast to IL-4, functional roles of other molecules produced by basophils in immune responses remain ill defined.
Both basophils and mast cells contain cytoplasmic granules that are stained violet with basic aniline dyes, as described first by Paul Ehrlich more than 130 years ago. These granules store preformed materials such as histamine and proteases, and release them upon activation.1,2 Ten serine proteases have been identified in murine mast cells, including 6 chymases (mouse mast cell protease-1 [mMCP-1], mMCP-2, mMCP-4, mMCP-5, mMCP-9, mMCP-10) and 4 tryptases (mMCP-6, mMCP-7, mMCP-11, mouse transmembrane tryptase).10-14 mMCP-11 is the most recently discovered member of the mouse mast cell tryptase family and the most distantly related to other members in terms of the amino acid sequence.15 We previously demonstrated that mMCP-11 is preferentially expressed by basophils rather than mast cells, whereas mMCP-6 and mMCP-7 are expressed by mast cells but not basophils.16 None of the mast cell chymases are expressed by basophils, whereas the expression of granzyme B–like protease mMCP-8 is confined to basophils.16,17 Thus, even though basophils and mast cells possess apparently similar basophilic granules, the repertoire of serine proteases stored in the granules is distinct between them, suggesting that mMCP-8 and mMCP-11 might be involved in nonredundant functions of basophils. We previously reported that the subcutaneous injection of recombinant mMCP-11 induces edematous skin swelling with increased microvascular permeability, suggesting that mMCP-11 may contribute to the development of basophil-mediated inflammatory responses.18
The nonredundant role of basophils in vivo has been clearly demonstrated in previous studies.19-21 A single intradermal administration of allergens in the ear skin of IgE-sensitized mice induced 3 consecutive waves of ear swelling. The first 2 waves (early- and late-phase ear swelling on day 1) were mast cell–dependent, immediate allergic reactions. The third wave of ear swelling, which started on postchallenge day 2 and peaked on day 4, was accompanied by massive infiltration of eosinophils, neutrophils, monocytes, and macrophages in the ear skin lesion. We designated this delayed-onset response immunoglobulin E–mediated chronic allergic inflammation (IgE-CAI).19 Mast cells are dispensable for IgE-CAI, whereas basophil depletion prevents the development of IgE-CAI, even though basophils represented only 1% or 2% of infiltrates in the postchallenge skin lesion. Thus, basophils but not mast cells play an essential role in IgE-CAI.19 However, it remains to be determined how basophils elicit allergic inflammation in the IgE-CAI response.
In the present study, we generated a line of mMCP-11–deficient knockout (KO) mice to clarify the functional role of mMCP-11 in vivo. The KO mice showed an ameliorated IgE-CAI response, with reductions of swelling, vascular permeability, and leukocyte infiltration in the skin lesion, even though their basophil development remained unimpaired. The results of in vitro experiments suggested that mMCP-11–mediated proteolytic products of serum proteins, rather than mMCP-11 itself, trigger migration of leukocytes, including basophils, via 1 or more G protein–coupled receptors. Thus, the present study uncovered the crucial role of basophil tryptase mMCP-11 in allergic inflammation.
Materials and methods
Mice
C57BL/6 mice (7-9 weeks old) were purchased from Japan SLC. To generate mMCP-11–deficient mice, the targeting vector for replacing the exons 1, 2, and 3 of the Mcpt11 (Prss34) gene with the loxP-PGK-gb2-neo-loxP cassette (Gene Bridges) was constructed by using Red/ET recombination (Gene Bridges) and transfected into C57BL/6 ES cells (UNITECH). The resultant knockout (KO) mice were further crossed with CAG-Cre mice (provided by J. Miyazaki, Osaka University)22 to remove the loxP-flanked cassette from the Mcpt11 allele. All animal studies were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University.
Antibodies and recombinant mMCP-11
The following antibodies were purchased from BioLegend: biotinylated anti-CD49b; fluorescein isothiocyanate (FITC)–conjugated anti-CD49b, anti-F4/80; phycoerythrin (PE)–conjugated anti-FcεRIα, anti-CD45 (30-F11); allophycocyanin (APC)–conjugated anti-CD200R3, anti-Ly-6G; Pacific Blue–conjugated anti-CD117, anti-CD11b. PE-conjugated anti-Siglec-F (E50-2440) from BD Biosciences also was used. Anti-mMCP-11 and anti-mMCP-8 antibodies were obtained as reported previously.16 IgE (IGEL b4) specific to 2,4,6-trinitrophenol (TNP) and anti-CD16/anti-CD32 (2.4G2) were prepared in our laboratory. Recombinant mMCP-11 and its protease-dead mutant (in which Ala was substituted for Ser200) were prepared as described previously.18
Generation of bone marrow–derived basophils
Mouse bone marrow–derived basophils (BMBAs) were generated as described previously.16 Briefly, bone marrow cells were cultured in the presence of 300 pg/mL recombinant mouse IL-3 (BioLegend) for 7 days, followed by purification of CD49b+ cells by using the IMag cell separation system (BD Biosciences) with biotinylated anti-CD49b (monoclonal antibody DX5) and streptavidin-conjugated magnetic particles. The purity of c-kit-CD49b+CD200R3+ BMBAs was more than 95% after magnetic purification.
Induction of IgE-CAI and passive cutaneous anaphylaxis
IgE-CAI was induced as described previously.19 In brief, mice were sensitized with an intravenous injection of 300 μg anti-TNP IgE and challenged on the following day with an intradermal injection of 10 μg TNP-ovalbumin (OVA) into the right ear and OVA alone (the control) into the left ear. Ear thickness was measured at 24-hour intervals for 7 days, and thickness difference between the right and left ears was quantified (Δ = right ear − left ear). In some experiments, mice were treated with topical administration of 200 μg SC560, 200 μg meloxicam, 100 μg celecoxib, or vehicle (10 μL ethanol) alone once a day during the development of IgE-CAI. All 4 substances were administered topically. For Evans blue dye leakage analysis, mice were intravenously injected with 0.5% Evans blue dye in 100 μL phosphate-buffered saline (PBS) on day 3 of the IgE-CAI response. Two hours after the dye injection, ears were excised and incubated overnight in 0.7 mL formamide at 63°C to extract the dye that had leaked into the skin. The amount of dye in the extract was determined by a spectrophotometer set at 620 nm. Passive cutaneous anaphylaxis was induced as previously described with some modifications.23 Briefly, mice were locally sensitized with intradermal injections of 100 ng anti-TNP IgE and control PBS in the right and left ear, respectively. On the following day, mice were challenged with an intravenous injection of 200 μg TNP-OVA in 200 μL of PBS containing 0.25% Evans blue dye. Thirty minutes later, the treated ears were excised, and the dye was extracted and measured.
Flow cytometric, histopathological, and western blot analyses
Single cell suspensions were obtained from ear skins by treatment with 125 U/mL collagenase (Wako) at 37°C for 2 hours. After pretreatment with anti-CD16/32 (2.4G2 clone) and normal rat serum to avoid the nonspecific binding of irrelevant antibodies, cells were stained with the indicated antibody combination on ice for 30 minutes, and analyzed with a benchtop cytometer (FACSCanto, BD Biosciences). Cell lineages were defined as follows: basophils (c-kit−CD49b+CD200R3+), eosinophils (Siglec-F+SSChigh), neutrophils (Ly-6G+), monocyte-macrophages (F4/80+CD11b+ among cells in which both eosinophils and neutrophils were excluded). For histopathological examination, ear specimens were fixed and embedded in paraffin, and sections were stained with hematoxylin and eosin. For western blot analysis, BMBAs were lysed with a radioimmunoprecipitation assay buffer–containing protease inhibitor cocktail (Thermo Scientific), and total cell lysates were analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by immunoblot analysis with the indicated antibodies.
Quantitative PCR assay
Total RNA was extracted from tissues or isolated cells by using the RNeasy Mini Kit (QIAGEN), followed by complementary DNA (cDNA) synthesis with reverse transcription by using oligo-dT and random primers. Quantitative polymerase chain reaction (PCR) assay of cDNA was performed by using the following primer sets: 5′-CTGGCTCCTGTTCCTCAGTCT-3′ and 5′-GCTGTGCTCCATGTCGTAGAG-3′ for Mcpt11 (Prss34); 5′-CACTGGTCAATGACATCATGCTCC-3′ and 5′-GTCAGACAAAGTGCAATTGGCCAAC-3′ for Mcpt8; 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ for Gapdh. Relative gene expression levels were calculated by using standard curves generated by serial dilutions of each cDNA standard and normalized by Gapdh expression levels.
In vitro stimulation of basophils
BMBAs were sensitized with 1 μg/mL anti-TNP IgE overnight and then stimulated with 300 ng/mL (for degranulation assay) or 400 ng/mL (for measurement of cytokines) TNP-OVA or control OVA. Degranulation of BMBAs was determined by measurement of β-hexosaminidase release as previously described.24 For measurement of cytokine production, BMBAs sensitized with IgE were stimulated with antigens for 24 hours. The concentrations of IL-4 and IL-6 in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA MAX, BioLegend) and bead-based immunoassay (Cytometric Bead Array, BD Biosciences), respectively.
Transwell migration assay
Leukocytes used for this assay were prepared as follows. Neutrophils were prepared from the bone marrow by using 62% Percoll gradient separation as described previously.25 Eosinophils were isolated from the peritoneum of mice that had been treated for 7 days with daily intraperitoneal administration of IL-5.26 Macrophages were isolated from the peritoneum of mice that had been treated with intraperitoneal administration of 1 mL of 4% thioglycolate broth 3 days before. The basophils used were BMBAs. The transwell apparatus (Kurabo) consisted of upper and lower chambers separated by a membrane with a 3-μm (for neutrophils) or 5-μm (for other cell types) pore size. Leukocytes (5 × 105 cells) were placed into the upper chamber while 10 μg/mL recombinant mMCP-11, its protease-dead mutant, control bovine serum albumin (BSA), or a chemokine (100 ng/mL macrophage inflammatory protein 2 [MIP-2], 200 ng/mL eotaxin, or 200 ng/mL monocyte chemoattractant protein 1 [MCP-1]) was included in culture medium in the lower chamber. After 90 minutes (for neutrophils) or 2 hours (for other cell types) of incubation at 37°C, the number of cells that had migrated into the lower chamber was counted. In some experiments, cells were pretreated with 200 ng/mL (for BMBAs) or 100 ng/mL (for other cell types) of pertussis toxin (PTX, Sigma-Aldrich) for 1 hour before the transwell migration assay. mMCP-11 was treated with 10 μg/mL nafamostat (Sigma-Aldrich) or control dimethyl sulfoxide before and after 2-hour incubation of mMCP-11 in culture medium prior to the transwell migration assay. Recombinant tetramerized human β-tryptase was purchased from Promega. Bovine trypsin was from Sigma-Aldrich.
Statistical analysis
Statistical analysis was performed with an unpaired Student t test. A P value of less than .05 was considered statistically significant.
Results
Generation of mMCP-11–deficient mice
To explore the role of mMCP-11 in vivo, we established mMCP-11–deficient KO mice by targeting the exons 1, 2, and 3 of the mMCP-11 coding gene Prss34 (Figure 1A). The number of c-kit−CD49b+CD200R3+FcεRIα+ basophils detected in the bone marrow, spleen, and peripheral blood of KO mice was similar to that in wild-type (WT) mice (Figure 1B-C). The number of other hematopoietic cells, including mast cells, was also comparable between WT and KO mice (supplemental Figure 1, available on the Blood Web site). When cultured with IL-3, bone marrow cells isolated from WT and KO mice generated comparable numbers of c-kit−CD49b+CD200R3+ basophils (BMBAs) (supplemental Figure 2), suggesting unimpaired development of basophils in KO mice. The lack of mMCP-11 expression in basophils from KO mice was confirmed by both messenger RNA (mRNA) and protein levels (Figure 1D-E) while the expression of basophil-specific mMCP-8 remained intact in basophils from KO mice (Figure 1D-E). When stimulated with IgE plus antigens, BMBAs from KO mice degranulated (Figure 1F) and produced cytokines IL-4 and IL-6 (Figure 1G) as much as did BMBAs from WT mice. Moreover, WT and KO mouse BMBAs could migrate toward chemokine MIP-2 to a comparable extent (Figure 1H). Thus, the deletion of the Prss34 gene did not seem to have any adverse effect on basophil homeostasis or the FcεRI- or chemokine-mediated activation of basophils.
Generation of mMCP-11–deficient mice. (A) Diagram shows gene targeting for the generation of mMCP-11–deficient mice. (B) Charts show results of flow cytometric analysis of indicated surface markers on bone marrow cells isolated from wild-type (WT) and mMCP-11–deficient (KO) littermate mice. c-kit–negative cells were gated and displayed. (C) Graphs show the numbers of basophils in the bone marrow, spleen, and peripheral blood of WT and KO mice (mean ± standard deviation [SD], n = 3 mice each). (D) Graphs show results of quantitative PCR analysis for the expression of Mcpt11 and Mcpt8 mRNAs in c-kit−CD49b+CD200R3+ basophils isolated from the bone marrow of WT and KO mice. (E) Western blot analysis for the expression of indicated proteins in bone marrow–derived basophils (BMBAs) generated from WT and KO mice. (F-G) BMBAs generated from WT (gray bars) and KO (black bars) mice were sensitized with anti-TNP IgE and then stimulated with TNP-OVA or control OVA. In panel F, the extent of degranulation was examined by using a β-hexosaminidase release assay (mean ± SD, n = 3 wells). In panel G, the concentration of cytokines IL-4 and IL-6 in culture supernatants was measured by using ELISA and bead-based immunoassay, respectively (mean ± SD, n = 3 wells). (H) Migration ability of WT (gray bars) and KO (black bars) BMBAs in response to chemokine MIP-2 (100 ng/mL) or control PBS was examined by using a transwell migration assay. The number of cells that migrated from the upper to lower chamber during 2-hour culture was counted (mean ± SD, n = 3 chambers each). Data shown in panels B-H are representative of at least 3 independent experiments. n.s., not statistically significant.
Generation of mMCP-11–deficient mice. (A) Diagram shows gene targeting for the generation of mMCP-11–deficient mice. (B) Charts show results of flow cytometric analysis of indicated surface markers on bone marrow cells isolated from wild-type (WT) and mMCP-11–deficient (KO) littermate mice. c-kit–negative cells were gated and displayed. (C) Graphs show the numbers of basophils in the bone marrow, spleen, and peripheral blood of WT and KO mice (mean ± standard deviation [SD], n = 3 mice each). (D) Graphs show results of quantitative PCR analysis for the expression of Mcpt11 and Mcpt8 mRNAs in c-kit−CD49b+CD200R3+ basophils isolated from the bone marrow of WT and KO mice. (E) Western blot analysis for the expression of indicated proteins in bone marrow–derived basophils (BMBAs) generated from WT and KO mice. (F-G) BMBAs generated from WT (gray bars) and KO (black bars) mice were sensitized with anti-TNP IgE and then stimulated with TNP-OVA or control OVA. In panel F, the extent of degranulation was examined by using a β-hexosaminidase release assay (mean ± SD, n = 3 wells). In panel G, the concentration of cytokines IL-4 and IL-6 in culture supernatants was measured by using ELISA and bead-based immunoassay, respectively (mean ± SD, n = 3 wells). (H) Migration ability of WT (gray bars) and KO (black bars) BMBAs in response to chemokine MIP-2 (100 ng/mL) or control PBS was examined by using a transwell migration assay. The number of cells that migrated from the upper to lower chamber during 2-hour culture was counted (mean ± SD, n = 3 chambers each). Data shown in panels B-H are representative of at least 3 independent experiments. n.s., not statistically significant.
mMCP-11 KO mice show ameliorated IgE-CAI response with the reduction in swelling and microvascular permeability of the skin
We previously reported that basophils crucially contribute to the development of IgE-CAI in the ear skin,19 and that basophil depletion abolishes the IgE-CAI response but not the mast cell–mediated immediate-type reaction.20 However, it remained to be determined what molecules derived from basophils were responsible for the development of IgE-CAI. To examine the role of mMCP-11 in IgE-CAI, WT and KO mice were passively sensitized with haptenic TNP-specific IgE and either challenged with intradermal injection of the corresponding allergen TNP-OVA or given control OVA. In both KO and WT mouse strains, TNP-OVA induced ear swelling with a peak on day 4 postchallenge, but the extent of ear swelling in KO mice was approximately half that in WT mice (Figure 2A). Moreover, the increase of microvascular permeability in the skin lesion was significantly lower in KO mice (Figure 2B). The increased permeability in WT mice was suppressed by treatment with meloxicam (COX-2 inhibitor) but not SC560 (COX-1 inhibitor), whereas neither drug significantly affected the permeability in KO mice (Figure 2C), suggesting that mMCP-11 increases permeability in the skin lesion through COX-2. In accordance with this, IgE-CAI ear swelling in WT but not KO mice was significantly inhibited by treatment with a COX-2 inhibitor, either meloxicam or celecoxib (Figure 2D and supplemental Figure 3). The tryptase activity of mMCP-11 remained unaffected by these inhibitors (data not shown). Thus, basophil-derived mMCP-11 appeared to contribute to ear swelling in the IgE-CAI response in part by means of COX-2-mediated vascularhyperpermeability.
mMCP-11–deficient mice show ameliorated IgE-CAI response in the skin. WT and KO mice were sensitized with anti-TNP IgE and challenged with intradermal administration of TNP-OVA or control OVA in their ear skin to induce IgE-CAI. (A) Time course of ear swelling (Δear thickness: TNP-OVA − OVA) is shown (mean ± SD, n = 4 mice each). (B) Three days after the allergen challenge, mice were treated with intravenous injection of Evans blue dye, and 2 hours later their ears were subjected to photography followed by the measurement of leaked dye (mean ± SD, n = 3 or 4 ears each). (C-D) WT and KO mice were treated once a day during IgE-CAI with topical administration of SC560, meloxicam, or vehicle (ethanol) alone. In panel C, mice were subjected to the Evans blue dye leakage analysis as in panel B. Change in Evans blue dye leakage (TNP-OVA − OVA) is shown (mean ± SD, n = 3 or 4 mice each). In panel D, time course of ear swelling (Δear thickness: TNP-OVA − OVA) is shown (mean ± SD, n = 6 mice each). Data shown in panels A-D are representative of at least 3 independent experiments. *P < .05; **P < .01; ***P < .001.
mMCP-11–deficient mice show ameliorated IgE-CAI response in the skin. WT and KO mice were sensitized with anti-TNP IgE and challenged with intradermal administration of TNP-OVA or control OVA in their ear skin to induce IgE-CAI. (A) Time course of ear swelling (Δear thickness: TNP-OVA − OVA) is shown (mean ± SD, n = 4 mice each). (B) Three days after the allergen challenge, mice were treated with intravenous injection of Evans blue dye, and 2 hours later their ears were subjected to photography followed by the measurement of leaked dye (mean ± SD, n = 3 or 4 ears each). (C-D) WT and KO mice were treated once a day during IgE-CAI with topical administration of SC560, meloxicam, or vehicle (ethanol) alone. In panel C, mice were subjected to the Evans blue dye leakage analysis as in panel B. Change in Evans blue dye leakage (TNP-OVA − OVA) is shown (mean ± SD, n = 3 or 4 mice each). In panel D, time course of ear swelling (Δear thickness: TNP-OVA − OVA) is shown (mean ± SD, n = 6 mice each). Data shown in panels A-D are representative of at least 3 independent experiments. *P < .05; **P < .01; ***P < .001.
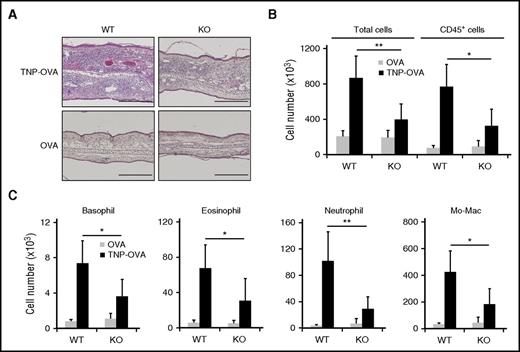
Cellular infiltration in the IgE-CAI skin lesion is decreased in KO mice
Histopathological examination of ear skin specimens indicated the reduction of cellular infiltration in the IgE-CAI skin lesion of KO mice compared with WT mice (Figure 3A). In accordance with this, flow cytometric analysis revealed that the number of total cells isolated from the skin lesion, mainly CD45+ hematopoietic cells, was reduced in KO mice to less than half that in WT mice (Figure 3B-C). Even though KO basophils by themselves showed unimpaired migration toward MIP-2 in vitro (Figure 1H), the number of basophils infiltrating the IgE-CAI skin lesion was significantly reduced in KO mice (Figure 3C). This was also the case for the numbers of eosinophils, neutrophils, and monocytes/macrophages in the skin lesion of KO mice (Figure 3C). Thus, mMCP-11 appeared to be involved in the infiltration and accumulation of these leukocytes in the IgE-CAI skin lesion.
Cellular infiltration in the IgE-CAI skin lesion is decreased in mMCP-11–deficient mice. WT and KO mice were treated as in Figure 2 to induce IgE-CAI. (A) Photomicrographs show hematoxylin and eosin–stained specimens of IgE-CAI skin lesions on day 3 postchallenge. Scale bars, 200 μm. (B-C) Graphs show the numbers of cells of each indicated cell type that were isolated from the TNP-OVA–treated ear skin (black bars) or control OVA–treated ear skin (gray bars) on day 3 postchallenge (mean ± SD, n = 5 or 6 ears each). Data shown are representative of 3 independent experiments. *P < .05; **P < .01; Mo-Mac, monocytes/macrophages.
Cellular infiltration in the IgE-CAI skin lesion is decreased in mMCP-11–deficient mice. WT and KO mice were treated as in Figure 2 to induce IgE-CAI. (A) Photomicrographs show hematoxylin and eosin–stained specimens of IgE-CAI skin lesions on day 3 postchallenge. Scale bars, 200 μm. (B-C) Graphs show the numbers of cells of each indicated cell type that were isolated from the TNP-OVA–treated ear skin (black bars) or control OVA–treated ear skin (gray bars) on day 3 postchallenge (mean ± SD, n = 5 or 6 ears each). Data shown are representative of 3 independent experiments. *P < .05; **P < .01; Mo-Mac, monocytes/macrophages.
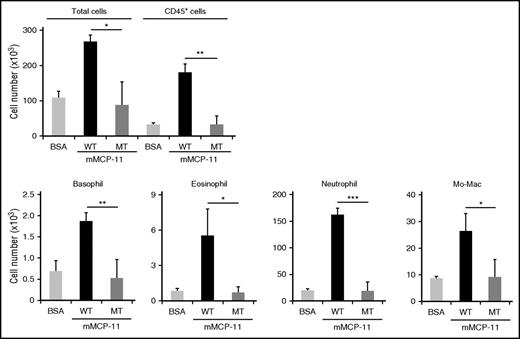
We previously reported that 1 single cutaneous injection of 10 μg recombinant mMCP-11 elicited transient ear swelling with increased microvascular permeability, whereas no significant infiltration of leukocytes was detected in the injection site.18 We assumed that this difference between the IgE-CAI skin lesion and the mMCP-11–injected skin could be attributed to different duration of mMCP-11 action. In contrast to the single injection, mMCP-11 likely accumulates in the IgE-CAI skin lesion because basophils are continuously recruited there and activated by allergens to release mMCP-11. To mimic this situation in vivo, we treated WT mice with repeated intradermal injection of recombinant mMCP-11 in the ear skin. Three injections of mMCP-11 administered at 24-hour intervals elicited the infiltration and accumulation of neutrophils, eosinophils, basophils, and monocytes/macrophages (Figure 4) along with transient ear swelling induced by each single injection (supplemental Figure 4A). The severity of mMCP-11–induced ear swelling was not affected by depletion of basophils, demonstrating that exogenous mMCP-11 can induce this reaction in a basophil-independent manner (supplemental Figure 4A-B). It is important that mMCP-11 carrying a mutation in the active site of tryptase did not show such a leukocyte recruiting ability (Figure 4), indicating that the protease activity of mMCP-11 is essential for this function.
Repeated intradermal administration of recombinant mMCP-11 in the ear skin induces leukocyte infiltration in a protease activity–dependent manner. WT mice were treated 3 times at 24-hour intervals with intradermal administration of 10 μg WT mMCP-11 (black bars), inactive mutant (MT) mMCP-11 (dark gray bars), or control BSA (light gray bars) each time. The numbers of cells (total, CD45+ cells, and indicated leukocyte types) isolated from the ear skin at 4 hours postchallenge are shown (mean ± SD, n = 3 ears each). Data shown are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Repeated intradermal administration of recombinant mMCP-11 in the ear skin induces leukocyte infiltration in a protease activity–dependent manner. WT mice were treated 3 times at 24-hour intervals with intradermal administration of 10 μg WT mMCP-11 (black bars), inactive mutant (MT) mMCP-11 (dark gray bars), or control BSA (light gray bars) each time. The numbers of cells (total, CD45+ cells, and indicated leukocyte types) isolated from the ear skin at 4 hours postchallenge are shown (mean ± SD, n = 3 ears each). Data shown are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Proteolytic products generated by mMCP-11 elicit leukocyte migration via a G protein–coupled receptor in vitro
To explore mechanisms underlying the mMCP-11–mediated leukocyte recruitment, we used the transwell migration assay to examine the possibility that mMCP-11 can directly stimulate leukocyte migration. Basophils, eosinophils, neutrophils, and macrophages were individually placed in the upper chamber of the apparatus, while mMCP-11 was included in the lower chamber. Enzymatically active but not inactive mMCP-11 elicited transwell migration of eosinophils, basophils, and macrophages but not neutrophils (Figure 5A), demonstrating that the protease activity of mMCP-11 is essential for triggering their migration. In contrast, no significant migration of T or B cells was detected under these conditions (supplemental Figure 5). Moreover, the addition of meloxicam to the transwell culture produced no significant effect on mMCP-11–induced basophil migration (data not shown), suggesting that mMCP-11 elicited leukocyte migration in a COX-2–independent manner. Of note, the pretreatment of eosinophils, basophils, and macrophages with pertussis toxin almost completely abolished the mMCP-11–induced migration (Figure 5B). These results suggested that mMCP-11 functioned as a protease and triggered leukocyte migration through 1 or more G protein–coupled receptors.
mMCP-11 induces leukocyte migration in vitro. The ability of mMCP-11 to induce leukocyte migration was examined by using a transwell migration assay. Indicated types of cells (5 × 105 cells) were placed in the upper chamber while WT mMCP-11, MT mMCP-11, control BSA, or the indicated chemokine was included in culture medium in the lower chamber. (A) The number of cells recovered from the lower chamber after 1.5 hours (for neutrophils) or 2 hours (for other cell types) of incubation at 37°C is shown (mean ± SD, n = 3 chambers each). (B) Indicated types of cells were pretreated with pertussis toxin (PTX, gray bars) or control PBS (black bars) for 1 hour previous to the transwell migration assay and analyzed as in panel A (mean ± SD, n = 3 chambers each). Data shown are representative of at least 3 independent experiments. **P < .01; ***P < .001.
mMCP-11 induces leukocyte migration in vitro. The ability of mMCP-11 to induce leukocyte migration was examined by using a transwell migration assay. Indicated types of cells (5 × 105 cells) were placed in the upper chamber while WT mMCP-11, MT mMCP-11, control BSA, or the indicated chemokine was included in culture medium in the lower chamber. (A) The number of cells recovered from the lower chamber after 1.5 hours (for neutrophils) or 2 hours (for other cell types) of incubation at 37°C is shown (mean ± SD, n = 3 chambers each). (B) Indicated types of cells were pretreated with pertussis toxin (PTX, gray bars) or control PBS (black bars) for 1 hour previous to the transwell migration assay and analyzed as in panel A (mean ± SD, n = 3 chambers each). Data shown are representative of at least 3 independent experiments. **P < .01; ***P < .001.
To clarify how the protease activity of mMCP-11 contributes to leukocyte migration, we added a protease inhibitor, nafamostat, to the mMCP-11–containing culture medium at the different timing in the transwell migration assay (Figure 6A). As expected, the migration of basophils, eosinophils, and macrophages was strongly reduced to a level as low as 10% to 15% of the control level (mMCP-11 alone) when nafamostat was added simultaneously with mMCP-11 to the lower chamber (compare experiments 1 and 2 in Figure 6A). In contrast, when nafamostat was added to the lower chamber to inactivate mMCP-11 after the culture medium had been incubated with mMCP-11 at 37°C for 2 hours previous to the transwell migration assay, leukocyte migration was elicited at a level as much as 60% to 75% of the control standard (experiment 3 in Figure 6A). In addition, serum depletion from culture medium completely abolished the basophil migration induced by mMCP-11 but not that induced by MIP-2 (Figure 6B). These results strongly suggested that mMCP-11 acted on a serum protein or proteins present in culture medium, and its proteolytic product rather than mMCP-11 itself promoted leukocyte migration. The lesser extent of migration in experiment 3 compared with experiment 1 could be attributed to the degradation of proteolytic products and lack of new products during the transwell migration assay due to the nafamostat-mediated inactivation of mMCP-11 in experiment 3. Importantly, the leukocyte migration in experiment 3 was abolished by the pretreatment of leukocytes with pertussis toxin (Figure 6C), implying that proteolytic products generated by mMCP-11 induced leukocyte migration via a G protein–coupled receptor.
Proteolytic products generated by mMCP-11 induce leukocyte migration via 1 or more G protein–coupled receptors. (A) The effect of nafamostat on mMCP-11–elicited leukocyte migration was examined in the transwell migration assay. mMCP-11 was added to the lower chamber together with nafamostat (experiment 2 [Exp2]) or control vehicle dimethyl sulfoxide alone (experiment 1 [Exp1]), and then the upper chamber containing leukocytes was placed onto the lower chamber to start the migration assay. In experiment 3 (Exp3), nafamostat was added to the lower chamber after the culture medium in the lower chamber had been incubated with mMCP-11 at 37°C for 2 hours prior to the transwell migration assay. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). (B) The transwell migration assay of BMBAs was performed by using a culture medium that was either serum sufficient (including 10% fetal calf serum) or serum deficient (including 0.1% BSA) in both upper and lower chambers. The number of cells that had migrated into the lower chamber after 2-hour incubation at 37°C is shown (mean ± SD, n = 3 chambers each). (C) As in experiment 3, nafamostat was added to the lower chamber after the culture medium had been incubated with mMCP-11 for 2 hours prior to the transwell migration assay, while the indicated types of cells were pretreated with PTX or control PBS for 1 hour and transferred to the upper chamber. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). Data shown in panels AC are representative of at least 3 independent experiments. **P < .01; ***P < .001.
Proteolytic products generated by mMCP-11 induce leukocyte migration via 1 or more G protein–coupled receptors. (A) The effect of nafamostat on mMCP-11–elicited leukocyte migration was examined in the transwell migration assay. mMCP-11 was added to the lower chamber together with nafamostat (experiment 2 [Exp2]) or control vehicle dimethyl sulfoxide alone (experiment 1 [Exp1]), and then the upper chamber containing leukocytes was placed onto the lower chamber to start the migration assay. In experiment 3 (Exp3), nafamostat was added to the lower chamber after the culture medium in the lower chamber had been incubated with mMCP-11 at 37°C for 2 hours prior to the transwell migration assay. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). (B) The transwell migration assay of BMBAs was performed by using a culture medium that was either serum sufficient (including 10% fetal calf serum) or serum deficient (including 0.1% BSA) in both upper and lower chambers. The number of cells that had migrated into the lower chamber after 2-hour incubation at 37°C is shown (mean ± SD, n = 3 chambers each). (C) As in experiment 3, nafamostat was added to the lower chamber after the culture medium had been incubated with mMCP-11 for 2 hours prior to the transwell migration assay, while the indicated types of cells were pretreated with PTX or control PBS for 1 hour and transferred to the upper chamber. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). Data shown in panels AC are representative of at least 3 independent experiments. **P < .01; ***P < .001.
Discussion
Previous study illustrated that basophils and mast cells display distinct repertoires of serine proteases stored in secretory granules.16 Among the tryptase members of the mMCP family, mMCP-11 is preferentially expressed by basophils instead of mast cells, whereas mMCP-6 and mMCP-7 are expressed only by mast cells in mice.16 Extensive investigation of mMCP-6 and mMCP-7 demonstrated that they play important roles in protective immunity against bacterial and parasitic infections27,28 and also in the development of inflammatory diseases such as autoimmune arthritis29,30 and colitis.31 In contrast, the functional significance of basophil tryptase mMCP-11 remained enigmatic. We showed in the present study that mMCP-11 is a crucial effector molecule in the development of IgE-CAI. This is the first demonstration that the basophil-derived protease plays a significant role in vivo.
mMCP-11–deficient KO mice showed much less severe IgE-CAI than did WT mice, characterized by milder ear swelling with reduced microvascular hyperpermeability and reduced leukocyte infiltration. We previously reported that basophil ablation during the progress of IgE-CAI resulted in drastic reduction of eosinophils and neutrophils infiltrating the skin lesion concomitantly with basophil elimination, suggesting the possible contribution of basophils to the eosinophil and neutrophil infiltration.20,21 The present study clearly demonstrated that basophil-derived mMCP-11 induces the infiltration of leukocytes, including basophils, eosinophils, neutrophils, and macrophages. Basophil infiltration in the IgE-CAI skin lesion was observed even in KO mice, albeit to a lesser extent than in WT mice. Therefore, mMCP-11 is not essential for basophil infiltration in IgE-CAI. Our findings instead suggest the following scenario: when basophils migrate into the skin lesion and are activated by allergens, they release mMCP-11, which in turn recruits more and more basophils together with other leukocytes. If this is the case, the ablation of mMCP-11 in basophils would reduce the infiltration of leukocytes, including basophils, just as we observed in the KO mice.
The detailed molecular mechanism by which mMCP-11 triggers leukocyte migration remains to be determined in future studies, as does the mechanism for mast cell tryptase– and chymase-induced chemotaxis of leukocytes.32-35 Nevertheless, our findings in the present study showed that the protease activity of mMCP-11 is essential for leukocyte migration and that a G protein-coupled receptor or receptors expressed on leukocytes is also involved. mMCP-11 does not seem to directly act on leukocytes to induce their migration. Most likely, mMCP-11 cleaves a serum protein, and its proteolytic product attracts basophils, eosinophils, and macrophages via a G protein–coupled receptor. In contrast, no significant migration of neutrophils was induced by mMCP-11 in the transwell migration assay, whereas intradermal administration of mMCP-11 elicited neutrophil infiltration in the skin. In the latter case, quantitative PCR analysis showed slightly upregulated expression of neutrophil-attracting chemokines such as CXCL1 and CXCL2 but without statistical significance (data not shown). The possible contribution of mMCP-11–elicited production of chemokines in the skin lesion to the migration of neutrophils and other leukocytes needs to be further explored.
mMCP-11 is highly expressed by all basophils and by a small subset of mast cells to a much lesser extent.16 As for the case of basophils, the number of mast cells in the skin and peritoneum of KO mice was comparable to that of WT mice (supplemental Figure 1), suggesting the unimpaired development of mast cells in the absence of mMCP-11. Moreover, the extent of microvascular leakage in the IgE-mediated passive cutaneous anaphylaxis was comparable between KO and WT mice (supplemental Figure 6), indicating intact degranulation of mast cells in KO mice. In accordance with this finding, mast cell–dependent, immediate-type (early- and late-phase) ear swelling ahead of the IgE-CAI response was normally elicited in KO mice (data not shown), in contrast to the amelioration of IgE-CAI. Thus, mMCP-11 did not appear to contribute to the mast cell–mediated immediate-type reaction we observed. However, the functional significance of mMCP-11 in mast cell–mediated chronic inflammation, if any, remains to be investigated.
The hSPL-4 gene on human chromosome 16p13.3 has been identified as the human ortholog of the mMCP-11 gene located on mouse chromosome 17A3.3.15 However, the hSPL-4 gene possesses a premature translational-termination codon and, unlike the mMCP-11 gene, does not encode production of enzymatically active enzyme. Of note, human basophils express α- and β-tryptases, albeit to a lesser extent than do mast cells,36,37 suggesting that α- and β-tryptases in human basophils might be functional orthologs of mMCP-11. Indeed, repeated intradermal injections of human β-tryptase elicited leukocyte infiltration into the skin of mice (supplemental Figure 7). In addition, β-tryptase induced transwell migration of basophils in vitro via a G protein–coupled receptors in a manner dependent on its protease activity and on the presence of serum (supplemental Figures 8 and 9), as did mMCP-11. It is noteworthy that trypsin, although it demonstrated higher activity than did β-tryptase and mMCP-11 when assessed by using a standard tryptase substrate (data not shown), showed little or no ability to induce basophil migration (supplemental Figure 8A), suggesting that not all tryptases share this property with mMCP-11.
In conclusion, the results of our study of mMCP-11–deficient mice demonstrate that basophil tryptase mMCP-11 plays a crucial role in the development of the IgE-CAI response through the promotion of microvascular leakage and leukocyte infiltration in a tryptase activity–dependent manner. This study has cast new light on the functional significance of proteases produced and released by basophils.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Miyazaki (Osaka University) for providing CAG-Cre mice.
This work was supported by research grants from Japan Science and Technology Agency (1A145) (H.K.) and the Japanese Ministry of Education, Culture, Sports, Science and Technology (15H05786 [H.K.] and 15K20969 [Y.Y.]).
Authorship
Contribution: M.I. and K.T. performed experiments; H.D. contributed to the initial study; M.F. and S.Y. generated mMCP-11–deficient mice; S.S. generated rmMCP-11; S.Y. provided helpful suggestions; M.I., Y.Y., and H.K. wrote the manuscript; and Y.Y. and H.K. designed and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshinori Yamanishi, Department of Immune Regulation, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan; e-mail: yamanishi.mbch@tmd.ac.jp.
References
Author notes
M.I. and K.T. contributed equally to this study.
Y.Y. and H.K. contributed equally to this study.

![Figure 1. Generation of mMCP-11–deficient mice. (A) Diagram shows gene targeting for the generation of mMCP-11–deficient mice. (B) Charts show results of flow cytometric analysis of indicated surface markers on bone marrow cells isolated from wild-type (WT) and mMCP-11–deficient (KO) littermate mice. c-kit–negative cells were gated and displayed. (C) Graphs show the numbers of basophils in the bone marrow, spleen, and peripheral blood of WT and KO mice (mean ± standard deviation [SD], n = 3 mice each). (D) Graphs show results of quantitative PCR analysis for the expression of Mcpt11 and Mcpt8 mRNAs in c-kit−CD49b+CD200R3+ basophils isolated from the bone marrow of WT and KO mice. (E) Western blot analysis for the expression of indicated proteins in bone marrow–derived basophils (BMBAs) generated from WT and KO mice. (F-G) BMBAs generated from WT (gray bars) and KO (black bars) mice were sensitized with anti-TNP IgE and then stimulated with TNP-OVA or control OVA. In panel F, the extent of degranulation was examined by using a β-hexosaminidase release assay (mean ± SD, n = 3 wells). In panel G, the concentration of cytokines IL-4 and IL-6 in culture supernatants was measured by using ELISA and bead-based immunoassay, respectively (mean ± SD, n = 3 wells). (H) Migration ability of WT (gray bars) and KO (black bars) BMBAs in response to chemokine MIP-2 (100 ng/mL) or control PBS was examined by using a transwell migration assay. The number of cells that migrated from the upper to lower chamber during 2-hour culture was counted (mean ± SD, n = 3 chambers each). Data shown in panels B-H are representative of at least 3 independent experiments. n.s., not statistically significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/25/10.1182_blood-2016-07-729392/4/m_blood729392f1.jpeg?Expires=1763470127&Signature=PNIgySbCPHwYWCjWxexP9J1G~qtBNAyD2~D~PrXbhDkSQMxPpXJ7nxOI~GInY5LKo3IrLlf2Sfdd4c0Qq0ogGAZPL28jKkqhcPDlPNNw~RzG3nJzmhBaKhqXpRHT4xloPX7MVaiwiIZ4l1NzoMiI6EdQbeZNY2u-wKZFDQknsebzqj-uIbOLNNj3I4k2fGjOHshrxba~FWreIjEAt5Imy8T7b-vMXDHZv-OKH2j6NGKTHNxShpcJGw-5Q8FlPWa1rrIuY2btqs03SiSVyN50XvZDs4pcVKfHGdfGmtj6OWbQFyzrWLZ9dbQ89-kQ8HyJ9CM7DAhq~zH4ur1TTLtDAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. Proteolytic products generated by mMCP-11 induce leukocyte migration via 1 or more G protein–coupled receptors. (A) The effect of nafamostat on mMCP-11–elicited leukocyte migration was examined in the transwell migration assay. mMCP-11 was added to the lower chamber together with nafamostat (experiment 2 [Exp2]) or control vehicle dimethyl sulfoxide alone (experiment 1 [Exp1]), and then the upper chamber containing leukocytes was placed onto the lower chamber to start the migration assay. In experiment 3 (Exp3), nafamostat was added to the lower chamber after the culture medium in the lower chamber had been incubated with mMCP-11 at 37°C for 2 hours prior to the transwell migration assay. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). (B) The transwell migration assay of BMBAs was performed by using a culture medium that was either serum sufficient (including 10% fetal calf serum) or serum deficient (including 0.1% BSA) in both upper and lower chambers. The number of cells that had migrated into the lower chamber after 2-hour incubation at 37°C is shown (mean ± SD, n = 3 chambers each). (C) As in experiment 3, nafamostat was added to the lower chamber after the culture medium had been incubated with mMCP-11 for 2 hours prior to the transwell migration assay, while the indicated types of cells were pretreated with PTX or control PBS for 1 hour and transferred to the upper chamber. The number of cells recovered from the lower chamber at the end of the migration assay is shown (mean ± SD, n = 3 chambers each). Data shown in panels AC are representative of at least 3 independent experiments. **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/25/10.1182_blood-2016-07-729392/4/m_blood729392f6.jpeg?Expires=1763470127&Signature=gVG3eeldrLbgkhcsVeAhA0BWNa9T~9LK2mo53P19IdpFTwlnrXK62E2V-lIW4IQcXCkjSlbODD3c3l8gTNoAbg2BpiDqnebc5G8XFvbjpaAjMAUmzq3KxdB8Xo8DTy7n2q3yy0LTDi~iDqZARvIrvZDXpGkvc99X56bnlIn17DkQoJKZG6htRZp73FUy6DPyTbM~a0l7mKYEDkW0KfPqUqafGFZHoO3uZ20cL5fG5M-wlNwKb3H9uVP6NoPfo19Pv4Riuuq9DpH9NOKVZwbnPYudhZcngrnkEIw5qFrutv7HqozmMyB2I0tbbtD3D~c98Rkm4JDFMU8mHC7d0awEEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal